
Draw the pyranose and furanose ring structure of glucose and fructose.
Answer
570.6k+ views
Hint:
The glucose structure was derived from a six closed ringed aldehyde structure called pyran. Fructose was derived from a six ringed ketone structure called furan. These structures were adopted after the previously theorised straight chain aldehyde and ketone did not match the properties of glucose and fructose.
Complete step by step answer:
The fischer structures of glucose and fructose that were first formulated failed as they did not contain common characteristics as that of the actual fructose and glucose compounds. The fischer structure of glucose and fructose represented the six carbon compounds as a straight chain compound. The bonds between the carbons were vertical.
The structure that was later introduced was the Haworth structures which represented glucose as a closed chain pyranose structure. This can be represented as follows:
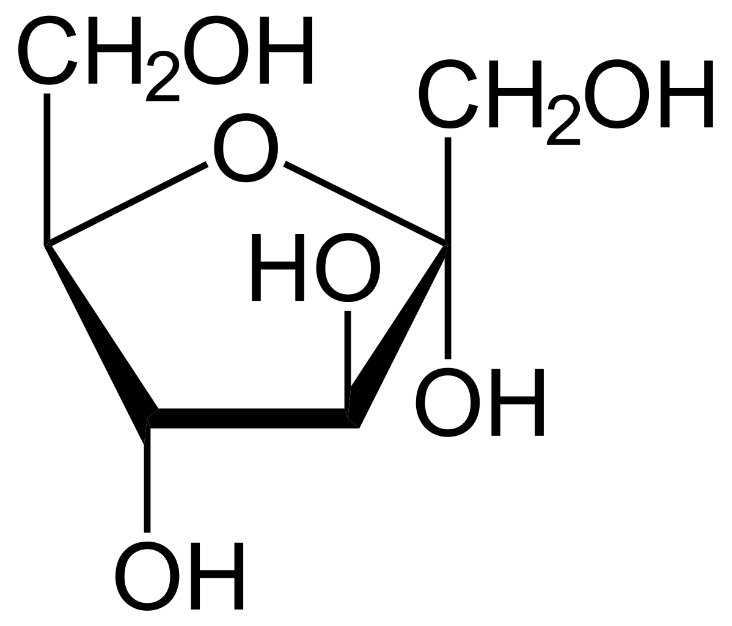
This structure showed that the carbon atoms of glucose and fructose were in one plane and the hydrogen atoms were perpendicular to the plane. The hydroxyl groups of all the carbons are placed outside the ring except for the third carbon in fructose and glucose.
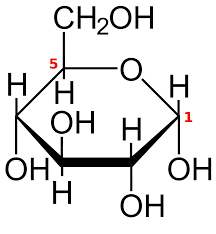
A number of experiments were carried out in order to prove that this structure is the correct structure.
These molecules are usually considered as the monomers of a carbohydrate chain since they are present in a single unit and are not attached to any other monosaccharide.
They are also present in carbohydrate chains to form larger molecules like mannose and galactose. Monosaccharides are the simplest unit of carbohydrates. They are the basis of formation of long chain carbohydrates like oligosaccharides and polysaccharides. They are sources of instant energy in the body and play an important role in carrying out the different activities in the body.
Note: Glucose and fructose are represented in closed rings known as Haworth structures. Fructose is derived from furan. Glucose is derived from pyran. They form monosaccharides that can link together to form larger and more complex compounds.
The glucose structure was derived from a six closed ringed aldehyde structure called pyran. Fructose was derived from a six ringed ketone structure called furan. These structures were adopted after the previously theorised straight chain aldehyde and ketone did not match the properties of glucose and fructose.
Complete step by step answer:
The fischer structures of glucose and fructose that were first formulated failed as they did not contain common characteristics as that of the actual fructose and glucose compounds. The fischer structure of glucose and fructose represented the six carbon compounds as a straight chain compound. The bonds between the carbons were vertical.
The structure that was later introduced was the Haworth structures which represented glucose as a closed chain pyranose structure. This can be represented as follows:

This structure showed that the carbon atoms of glucose and fructose were in one plane and the hydrogen atoms were perpendicular to the plane. The hydroxyl groups of all the carbons are placed outside the ring except for the third carbon in fructose and glucose.

A number of experiments were carried out in order to prove that this structure is the correct structure.
These molecules are usually considered as the monomers of a carbohydrate chain since they are present in a single unit and are not attached to any other monosaccharide.
They are also present in carbohydrate chains to form larger molecules like mannose and galactose. Monosaccharides are the simplest unit of carbohydrates. They are the basis of formation of long chain carbohydrates like oligosaccharides and polysaccharides. They are sources of instant energy in the body and play an important role in carrying out the different activities in the body.
Note: Glucose and fructose are represented in closed rings known as Haworth structures. Fructose is derived from furan. Glucose is derived from pyran. They form monosaccharides that can link together to form larger and more complex compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




