
Draw the structure of ${{C}_{6}}{{H}_{12}}$.
Answer
587.7k+ views
Hint: First find which type of functional group can be present in the compound and then assign the structure. If formula of a hydrocarbon compound is ${{C}_{n}}{{H}_{2n+2}}$, then it is alkane, if the formula is ${{C}_{n}}{{H}_{2n}}$, then it is alkene and if the formula is ${{C}_{n}}{{H}_{2n-2}}$, then the compound is alkyne.
Complete step by step solution:
We are given the molecular formula of the compound and we will now draw its structure.
- We will need to identify the functional group present in the compound in order to draw its structure. See, there are only carbons and hydrogens present in the compound. So, this is a type of a hydrocarbon compound.
- We know that if the molecular formula of the compound is like ${{C}_{n}}{{H}_{2n}}$, then there is a possibility that the compound has an alkene functional group. In our compound, n is 6 and 2n is 12. So, this compound has an alkene functional group.
- Now, we will try to assign this molecule a structure and keep in mind that there is a C-C double bond present in the compound. So, one of the possible structures of this compound is as shown below.
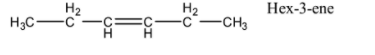
- Now, the double bond in the given stricture is on third and fourth carbon. It can be between any carbons and all those structures of the compounds are acceptable. Geometry at these double bonds can be either cis or trans.
Note: Here another possibility of a structure of ${{C}_{6}}{{H}_{12}}$ is a six member ring without any unsaturation. This compound is called cyclohexane and this does not have any double bond (alkene functional group) in its structure.
Complete step by step solution:
We are given the molecular formula of the compound and we will now draw its structure.
- We will need to identify the functional group present in the compound in order to draw its structure. See, there are only carbons and hydrogens present in the compound. So, this is a type of a hydrocarbon compound.
- We know that if the molecular formula of the compound is like ${{C}_{n}}{{H}_{2n}}$, then there is a possibility that the compound has an alkene functional group. In our compound, n is 6 and 2n is 12. So, this compound has an alkene functional group.
- Now, we will try to assign this molecule a structure and keep in mind that there is a C-C double bond present in the compound. So, one of the possible structures of this compound is as shown below.

- Now, the double bond in the given stricture is on third and fourth carbon. It can be between any carbons and all those structures of the compounds are acceptable. Geometry at these double bonds can be either cis or trans.
Note: Here another possibility of a structure of ${{C}_{6}}{{H}_{12}}$ is a six member ring without any unsaturation. This compound is called cyclohexane and this does not have any double bond (alkene functional group) in its structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




