
Draw the structure of Dicyclopropyl Methane showing all C and H atoms.
Answer
507.9k+ views
Hint: We know that the name of the structure of any compound in organic chemistry is determined by the rules of the nomenclature. It includes the long parent carbon chain rule, lowest number for the main functional group rule, maximum substituents rule and lowest number to the multiple bond rule.
Complete answer:
As we know that the valency of an atom is equal to the number of electrons present in the valence shell of the atom. The valence electrons are the most loosely held electrons in the atom and thus they determine the properties of the element. Each atom tends to attain a stable noble gas- like electronic configuration. Valency of an element represents the combining capacity of that element. Valency is not always necessarily equal to the number of valence electrons.
The IUPAC naming while naming the compound like the naming of functional groups, alkyl groups, chains, etc. So that we can easily draw the structure of the molecule by using its name. In the given question we must draw the structural formula of the given compound that is a Dicyclopropyl Methane molecule. IUPAC or International Union of Pure and Applied Compound is a method of naming any organic compound. Let us draw the structures of the given compounds with the help of rules of nomenclature as prescribed by the International Union of Pure and Applied Chemistry (IUPAC).
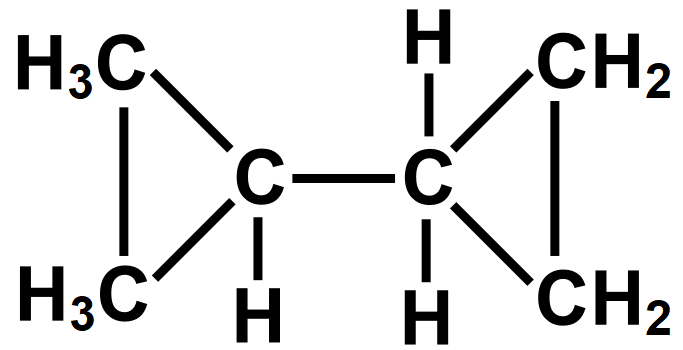
Note:
Remember that although the compound Dicyclopropylmethane has various numbers of isomers due to the different positioning of the bromine atom, the priority of drawing the compound. This stable electronic configuration can be attained by either losing, gaining, or sharing electrons.
Complete answer:
As we know that the valency of an atom is equal to the number of electrons present in the valence shell of the atom. The valence electrons are the most loosely held electrons in the atom and thus they determine the properties of the element. Each atom tends to attain a stable noble gas- like electronic configuration. Valency of an element represents the combining capacity of that element. Valency is not always necessarily equal to the number of valence electrons.
The IUPAC naming while naming the compound like the naming of functional groups, alkyl groups, chains, etc. So that we can easily draw the structure of the molecule by using its name. In the given question we must draw the structural formula of the given compound that is a Dicyclopropyl Methane molecule. IUPAC or International Union of Pure and Applied Compound is a method of naming any organic compound. Let us draw the structures of the given compounds with the help of rules of nomenclature as prescribed by the International Union of Pure and Applied Chemistry (IUPAC).

Note:
Remember that although the compound Dicyclopropylmethane has various numbers of isomers due to the different positioning of the bromine atom, the priority of drawing the compound. This stable electronic configuration can be attained by either losing, gaining, or sharing electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




