
Draw the structure of ethyl ethanoate
Answer
569.7k+ views
Hint: We can draw the structure of a given organic compound with the help of the IUPAC name of that compound. The IUPAC name of any organic compound is unique and only denotes one molecular structure.
Complete step by step answer:
Ethyl ethanoate is generally abbreviated as EtOAc, EA, or ETAC. In the case of the ethyl ethanoate, the carbonyl carbon atom is $s{p^2}$ hybridized and the geometry of the other part of the molecule is tetrahedral. The chemical formula for ethyl ethanoate is $C{H_3}COO{C_2}{H_5}$. The structure of the given compound i.e. ethyl ethanoate or ethyl acetate is given below:
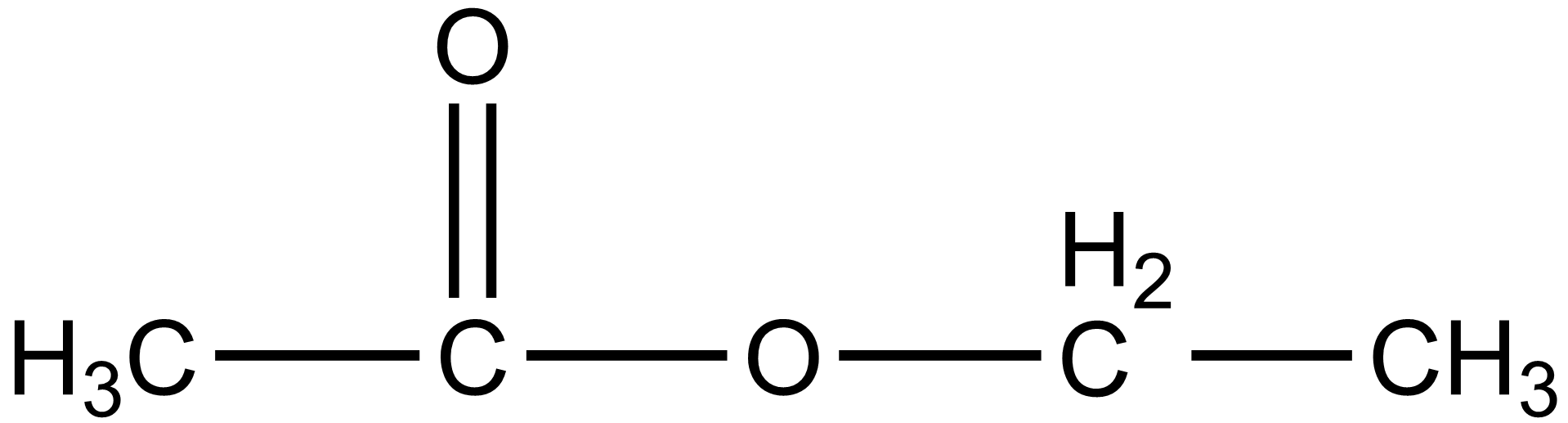
Additional information:
Ethyl ethanoate is generally prepared by gently heating the mixture of ethanoic acid, ethanol, and concentrated sulphuric acid either in a water bath or an electric heater. This reaction is known as esterification. The chemical reaction for the preparation of ethyl ethanoate is given as:
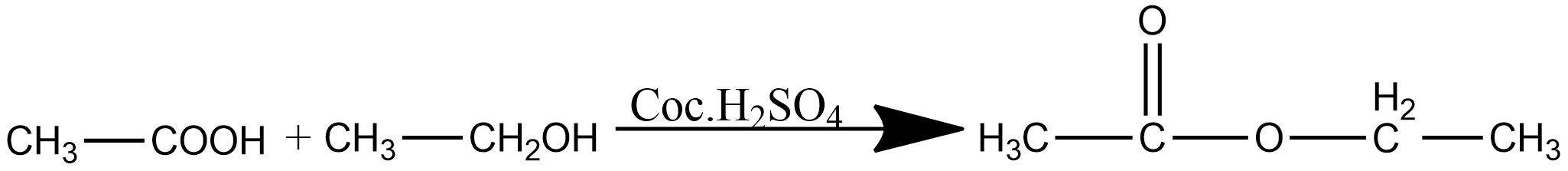
The ester formed during the above reaction is distilled off as soon as possible and cooled in a separate beaker by condensation. since the above reaction i.e. esterification reaction is reversible the ester formed is removed by distillation from the reaction mixture. By process of Esterification, we get many commonly used products such as biodiesel, pharmaceuticals, solvents of paints, adhesives, pesticides, etc.
Note: Ethyl ethanoate is an organic compound that is mainly used as a solvent in a different chemical reaction. Ethyl ethanoate is highly flammable and it is generally found in alcoholic drinks such as wines. It is a colorless and sweet-smelling ester.
Complete step by step answer:
Ethyl ethanoate is generally abbreviated as EtOAc, EA, or ETAC. In the case of the ethyl ethanoate, the carbonyl carbon atom is $s{p^2}$ hybridized and the geometry of the other part of the molecule is tetrahedral. The chemical formula for ethyl ethanoate is $C{H_3}COO{C_2}{H_5}$. The structure of the given compound i.e. ethyl ethanoate or ethyl acetate is given below:

Additional information:
Ethyl ethanoate is generally prepared by gently heating the mixture of ethanoic acid, ethanol, and concentrated sulphuric acid either in a water bath or an electric heater. This reaction is known as esterification. The chemical reaction for the preparation of ethyl ethanoate is given as:
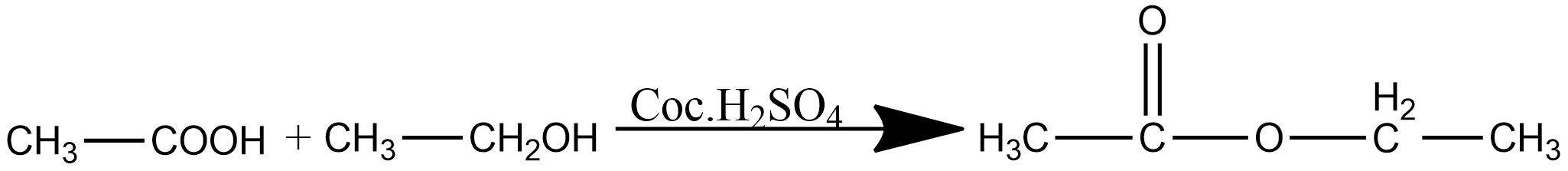
The ester formed during the above reaction is distilled off as soon as possible and cooled in a separate beaker by condensation. since the above reaction i.e. esterification reaction is reversible the ester formed is removed by distillation from the reaction mixture. By process of Esterification, we get many commonly used products such as biodiesel, pharmaceuticals, solvents of paints, adhesives, pesticides, etc.
Note: Ethyl ethanoate is an organic compound that is mainly used as a solvent in a different chemical reaction. Ethyl ethanoate is highly flammable and it is generally found in alcoholic drinks such as wines. It is a colorless and sweet-smelling ester.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




