
Draw the structure of \[{\text{hex - 1 - en - 3 - ol}}\] compound.
Answer
552.9k+ views
Hint:In organic chemistry, we have three different ways to draw organic molecules including structural formula, condensed formula, and skeletal structures (also known as line – bond structures or line formulas). \[{\text{hex - 1 - en - 3 - ol}}\]is an organic compound, to draw the structure format with root, prefix, and suffix.
Complete step by step answer:1) In the compound \[{\text{hex - 1 - en - 3 - ol}}\] parent chain has ${\text{6}}$ carbon atoms. As the name depicts the first carbon atom has a double bond while the third carbon atom has alcohol as a functional group \[{\text{ - OH}}\] as a substituent.
2) The structural formula of a compound displays the atoms of the molecule in which they are bonded, and also depicts how the atoms are bonded to one another.
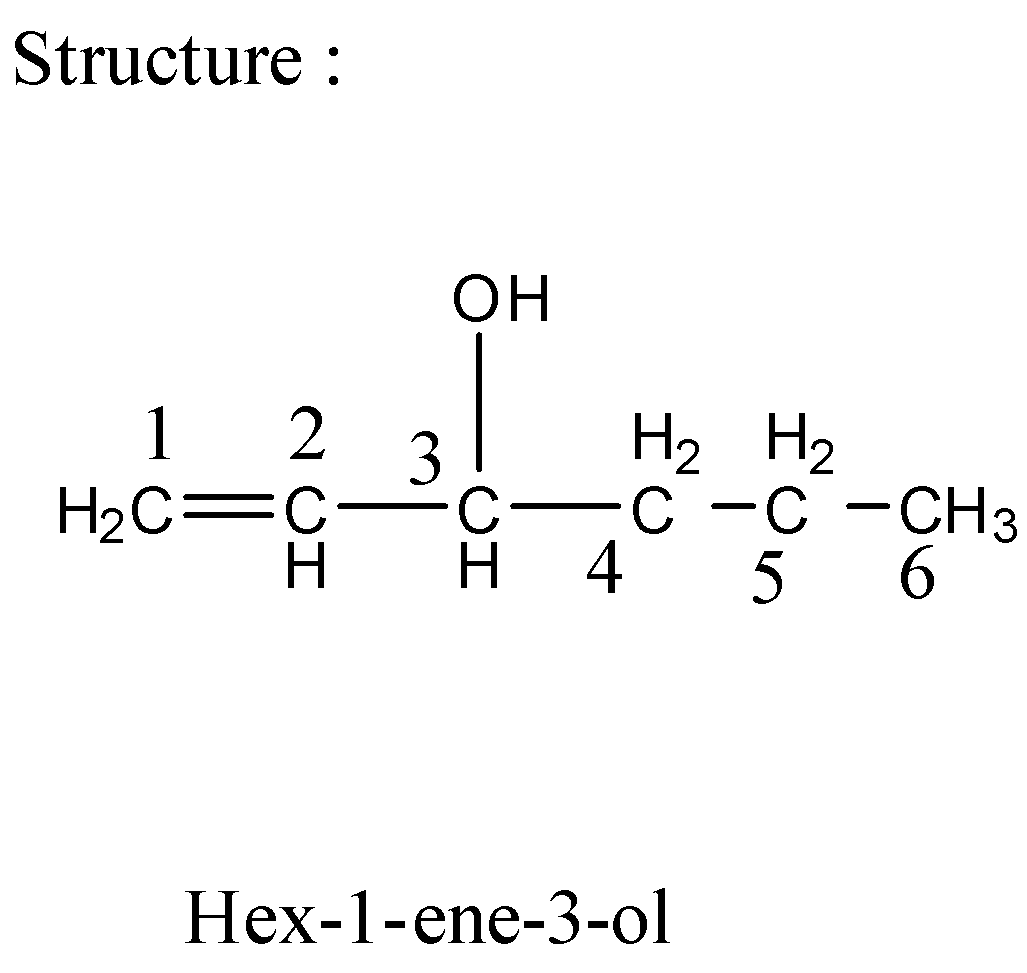
3) The condensed formula shows the order of atoms similar to the structural formula but is written in a single \[{C_6}{H_{12}}O\] line and makes it easier and faster to write out. The condensed formula also shows that a group of atoms is connected to a single atom in a compound. \[{C_6}{H_{12}}O\] has condensed Formula as \[{\text{hex - 1 - en - 3 - ol}}\].
4) The line formulas are used to write carbon and hydrogen atoms more significantly by replacing the letter "C" with lines. Carbon is present wherever a line intersects another line while the hydrogen atoms that are attached to elements other than carbon are shown. These represent the structure to be the same as a structural formula.
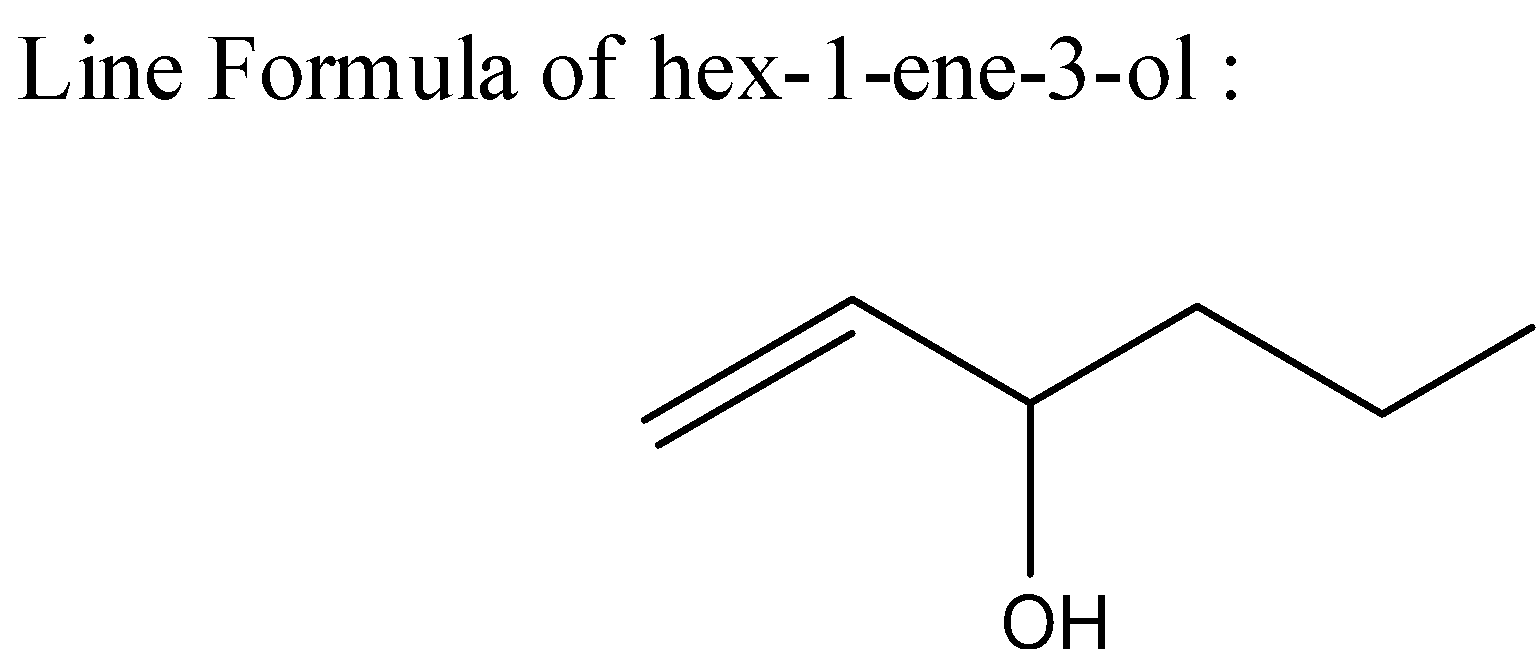
Note:
For an organic compound it is very important to draw structural formulas because in most of the cases a molecular formula does not correctly represent a single compound. The structural formula, condensed formula, and line formula are just different ways to represent a chemical compound.
Complete step by step answer:1) In the compound \[{\text{hex - 1 - en - 3 - ol}}\] parent chain has ${\text{6}}$ carbon atoms. As the name depicts the first carbon atom has a double bond while the third carbon atom has alcohol as a functional group \[{\text{ - OH}}\] as a substituent.
2) The structural formula of a compound displays the atoms of the molecule in which they are bonded, and also depicts how the atoms are bonded to one another.

3) The condensed formula shows the order of atoms similar to the structural formula but is written in a single \[{C_6}{H_{12}}O\] line and makes it easier and faster to write out. The condensed formula also shows that a group of atoms is connected to a single atom in a compound. \[{C_6}{H_{12}}O\] has condensed Formula as \[{\text{hex - 1 - en - 3 - ol}}\].
4) The line formulas are used to write carbon and hydrogen atoms more significantly by replacing the letter "C" with lines. Carbon is present wherever a line intersects another line while the hydrogen atoms that are attached to elements other than carbon are shown. These represent the structure to be the same as a structural formula.
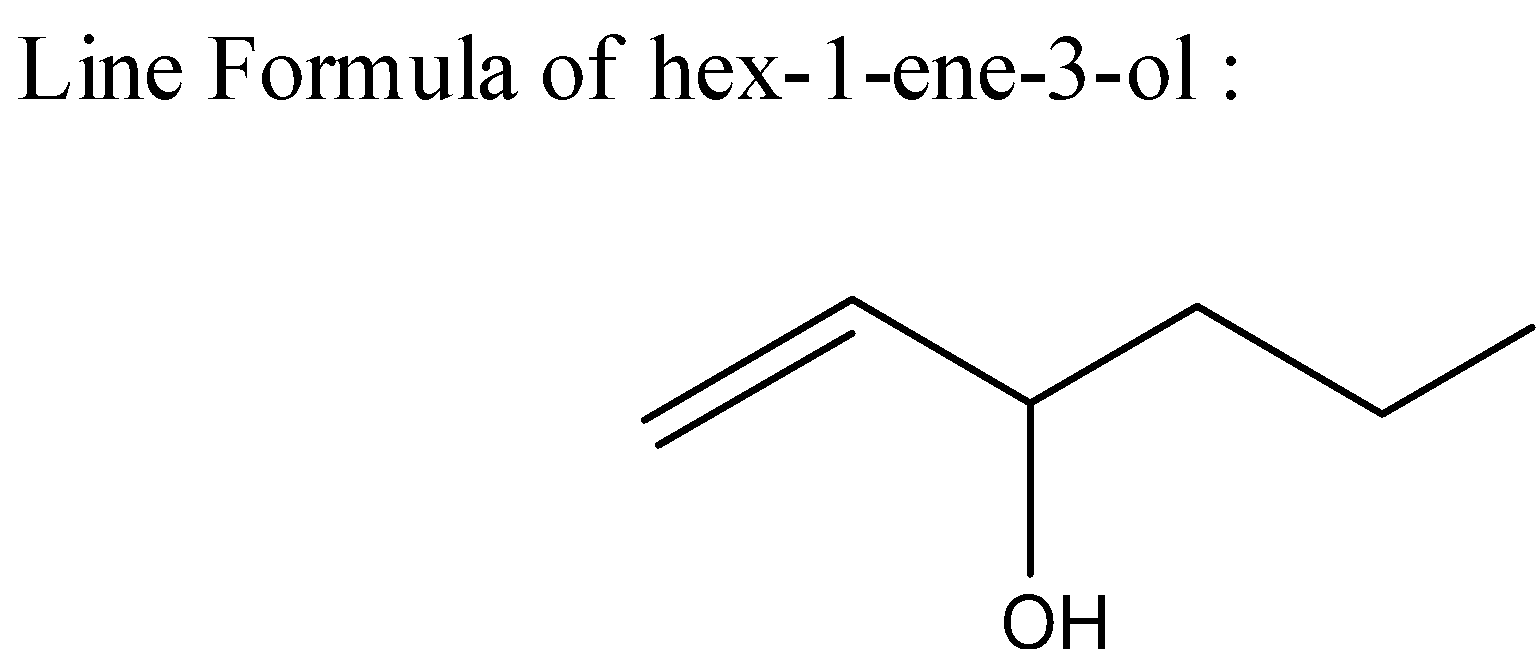
Note:
For an organic compound it is very important to draw structural formulas because in most of the cases a molecular formula does not correctly represent a single compound. The structural formula, condensed formula, and line formula are just different ways to represent a chemical compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




