
Draw the structure of $ {{P}_{4}}{{O}_{6}} $ and identify the number of single $ P-O $ bonds?
Answer
528.3k+ views
Hint: We know that the Bonds are usually formed by share or transfer of valence electrons. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interaction.
Complete step by step solution:
Bonds are formed when two or more atoms are able to satisfy the electron configuration stability of each other. This means that, in order to achieve maximum stability, valence orbitals in atoms must be fully filled or half filled. Also, the valence electron shell must fulfill the octet rule, i.e. there must be 8 electrons present in the valence shell. -Lone pairs of electrons are found in the valence shell of the atom. Lone pairs of electrons are basically the electron pairs that do not take part in the bond between two atoms. Compounds are formed due to the bonding of atoms. Bonding results in classification of valence electrons in bonding electrons and lone pairs.
$ {{P}_{4}}{{O}_{6}} $ (phosphorus trioxide) is also known as phosphorous oxide or phosphorous anhydride because it is the anhydride of orthophosphoric acid. It is a soft white solid. We know that since it is anhydride of an acid it will be acidic itself also and hydrolysis in water to form phosphorous acid. Phosphorus trioxide exists as a dimer and is written as $ {{P}_{4}}{{O}_{6}} $
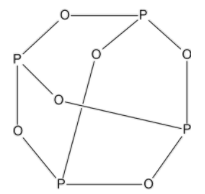
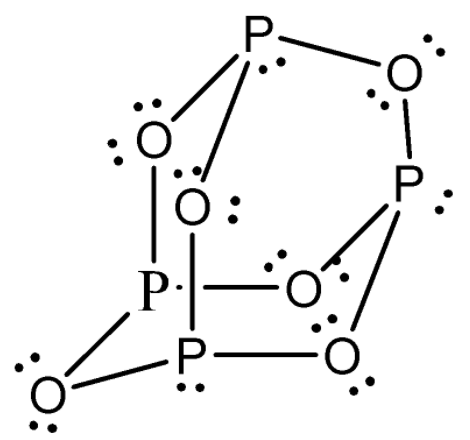
The structure of $ {{P}_{4}}{{O}_{6}} $ is given as:
Note:
Remember that the total number of valence electrons in any atom can be given by the sum of the number of bonding electrons and the number of lone pairs of electrons. In the case of neutral atoms, the number of valence electrons can be determined by the atom’s main group number. For instance, oxygen is in group six and therefore, has six valence electrons.
Complete step by step solution:
Bonds are formed when two or more atoms are able to satisfy the electron configuration stability of each other. This means that, in order to achieve maximum stability, valence orbitals in atoms must be fully filled or half filled. Also, the valence electron shell must fulfill the octet rule, i.e. there must be 8 electrons present in the valence shell. -Lone pairs of electrons are found in the valence shell of the atom. Lone pairs of electrons are basically the electron pairs that do not take part in the bond between two atoms. Compounds are formed due to the bonding of atoms. Bonding results in classification of valence electrons in bonding electrons and lone pairs.
$ {{P}_{4}}{{O}_{6}} $ (phosphorus trioxide) is also known as phosphorous oxide or phosphorous anhydride because it is the anhydride of orthophosphoric acid. It is a soft white solid. We know that since it is anhydride of an acid it will be acidic itself also and hydrolysis in water to form phosphorous acid. Phosphorus trioxide exists as a dimer and is written as $ {{P}_{4}}{{O}_{6}} $
The structure of $ {{P}_{4}}{{O}_{6}} $ is given as:


Note:
Remember that the total number of valence electrons in any atom can be given by the sum of the number of bonding electrons and the number of lone pairs of electrons. In the case of neutral atoms, the number of valence electrons can be determined by the atom’s main group number. For instance, oxygen is in group six and therefore, has six valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




