
Draw the structure of the compound:
${\text{4 - tert - butyl - 3 - iodoheptane}}$
Answer
552.9k+ views
Hint:In organic chemistry, we have three different ways to draw organic molecules including structural formula, condensed formula, and skeletal structures (also known as line – bond structures or line formulas). ${\text{4 - tert - butyl - 3 - iodoheptane}}$ is an organic halogen compound, to draw the structure format with root, prefix, and suffix.
Complete step by step answer:1) First of all, in the given compound the end word is -hept, which shows the longest carbon chain must have seven carbon atoms. There are two substituent presents in a structure, one at carbon atom number ${\text{3}}$ and one at ${\text{4}}$. The carbon numbered ${\text{3}}$ contains the substituent Iodine and the carbon number ${\text{4}}$ contains the substituent tert butyl.
2) The IUPAC name starts from the number of carbon atoms bearing the substituent followed by the name of the substituent. The substituents that are attached to the longest chain comes first as per alphabetical order and it is named first. Then the word root hept comes followed by the suffix -ane which means all the bonds are single bonds.
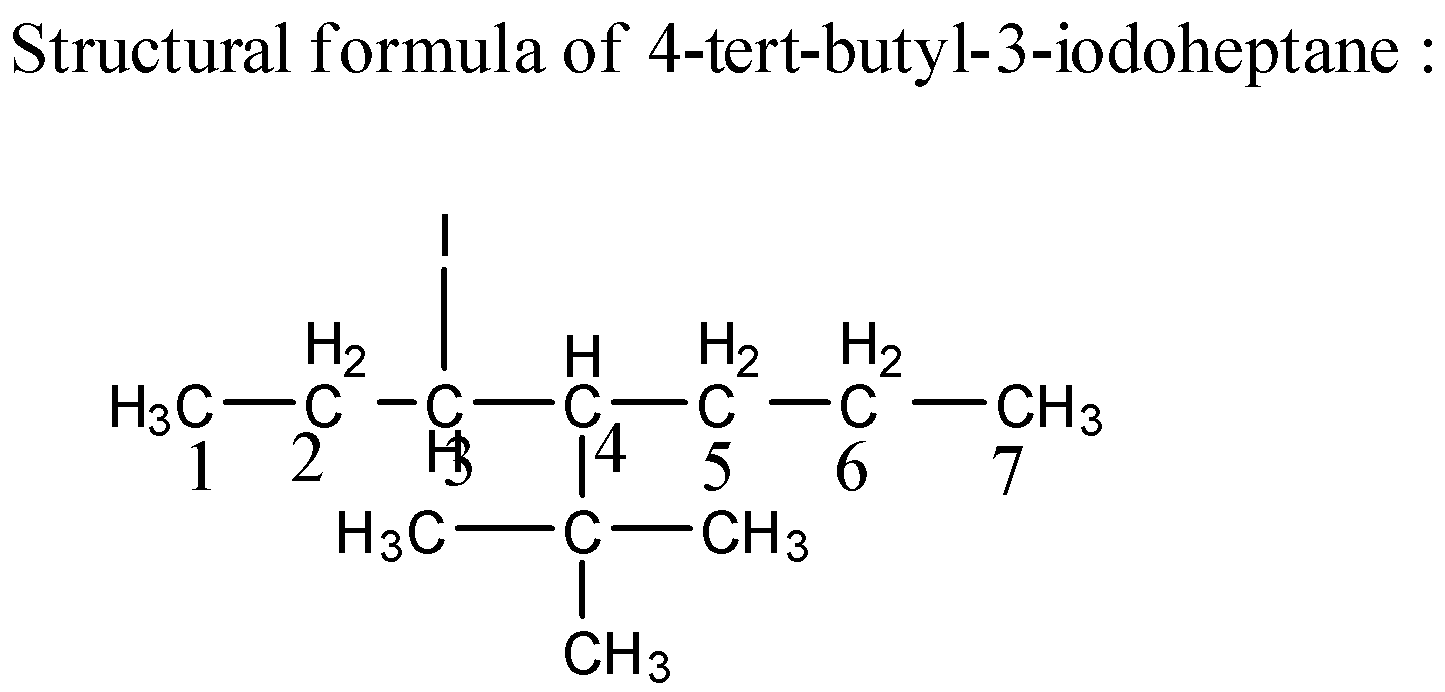
3) Structural formula- displays the atoms of the molecule in the order they are bonded.
Structural Formula of ${\text{4 - tert - butyl - 3 - iodoheptane}}$ is as below,
4) The condensed formula displays the order of atoms similar to the structural formula but is written in a single line and makes it easier and faster to write out. The condensed Formula of ${\text{4 - tert - butyl - 3 - iodoheptane}}$ is $C{H_3} - C{H_2} - CH(I) - C(CC{H_3}) - C{H_2} - C{H_2} - C{H_3}$
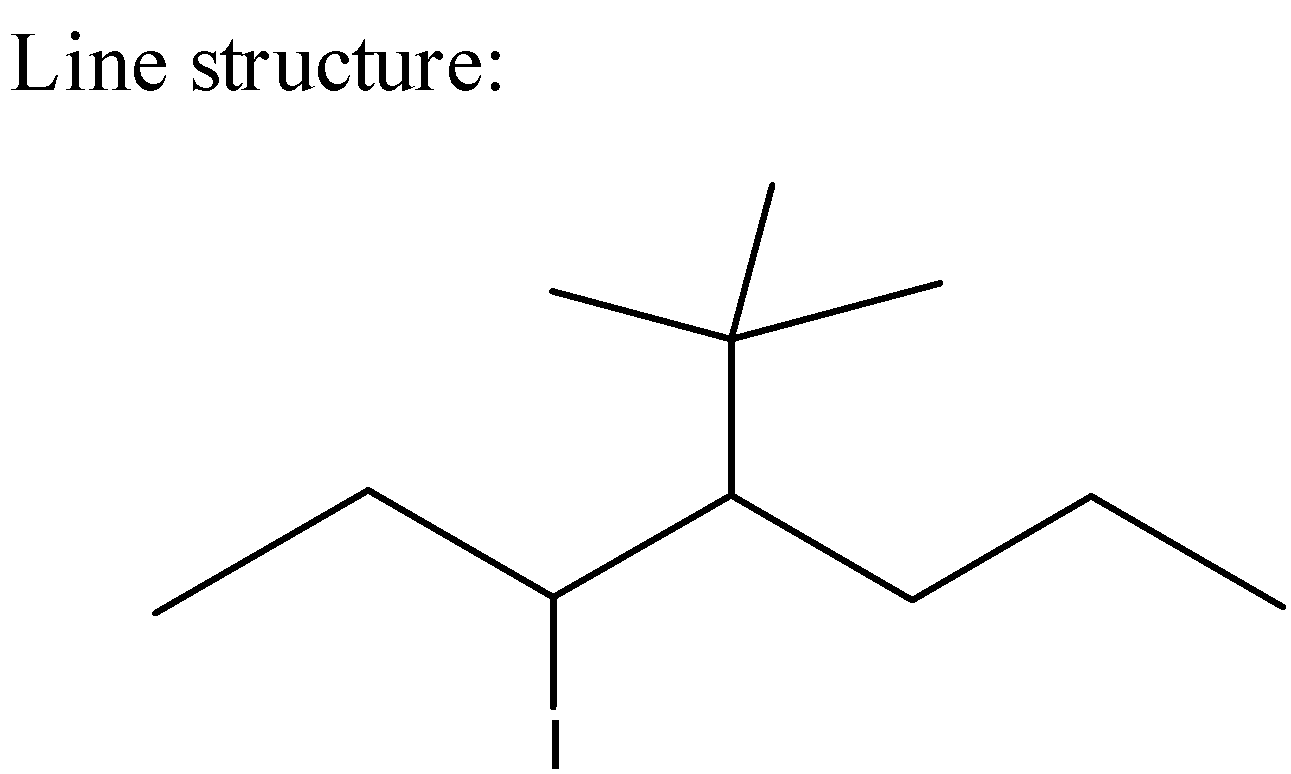
5) The line formulas are used to write carbon and hydrogen atoms more significantly by replacing the letter "C" with lines.
Line Formula is as below,
Note:
The numbering given in an IUPAC name shows the atoms or groups that are attached to the carbon atoms in the longest chain. It is very important to write a structure based on the IUPAC name as IUPAC naming gives the identity to a chemical structure.
Complete step by step answer:1) First of all, in the given compound the end word is -hept, which shows the longest carbon chain must have seven carbon atoms. There are two substituent presents in a structure, one at carbon atom number ${\text{3}}$ and one at ${\text{4}}$. The carbon numbered ${\text{3}}$ contains the substituent Iodine and the carbon number ${\text{4}}$ contains the substituent tert butyl.
2) The IUPAC name starts from the number of carbon atoms bearing the substituent followed by the name of the substituent. The substituents that are attached to the longest chain comes first as per alphabetical order and it is named first. Then the word root hept comes followed by the suffix -ane which means all the bonds are single bonds.
3) Structural formula- displays the atoms of the molecule in the order they are bonded.
Structural Formula of ${\text{4 - tert - butyl - 3 - iodoheptane}}$ is as below,

4) The condensed formula displays the order of atoms similar to the structural formula but is written in a single line and makes it easier and faster to write out. The condensed Formula of ${\text{4 - tert - butyl - 3 - iodoheptane}}$ is $C{H_3} - C{H_2} - CH(I) - C(CC{H_3}) - C{H_2} - C{H_2} - C{H_3}$
5) The line formulas are used to write carbon and hydrogen atoms more significantly by replacing the letter "C" with lines.
Line Formula is as below,

Note:
The numbering given in an IUPAC name shows the atoms or groups that are attached to the carbon atoms in the longest chain. It is very important to write a structure based on the IUPAC name as IUPAC naming gives the identity to a chemical structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




