
Draw the structure of the following
$HCl{O_3}$
Answer
564.6k+ views
Hint:First we have to know what is the central atom in the given compound and then what is the valency of the central atom, what is the hybridisation of the compound, what is the geometry and structure of the compound.
Complete answer:
So, starting from finding the central atom, here the central atom is chlorine, because if we try to make the bonds given, only chlorine will satisfy.
So, now the question is how many valence electrons in the chlorine atom or what is the valency of the chlorine, (because valency is the number of valence electrons present in the outermost valence shell).
Chlorine is having an atomic number of $17$ . Hence, the valency of the chlorine will be $7$ .Now, as chlorine has $7$ valence electrons, it makes $2$ double bonds with oxygen ( $2$ sigma and $2$ pi bonds), one with hydroxide ( $1$ sigma bond) leaving a lone pair on the chlorine atom.
So, the hybridisation of the compound will be $s{p^3}$ . So, the geometry of the compound will be tetrahedral. But due to the presence of a lone pair in the central atom the structure of the compound will be pyramidal as in the place of the one bond there is an lone pair, and we know that in the structure the lone pair is not shown. So, the final structure of the molecule would be pyramidal.
The structure is as shown in figure below:
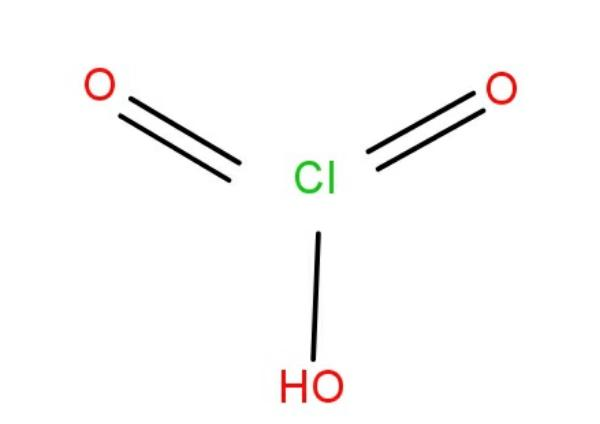
Note:
When drawing any structure keep the points in mind that the valency of the central atom is complete and presence of lone pair take part while writing geometry, but while drawing the structure it would not be shown. Due to the presence of lone pairs, the geometry of the compound will be distorted.
Complete answer:
So, starting from finding the central atom, here the central atom is chlorine, because if we try to make the bonds given, only chlorine will satisfy.
So, now the question is how many valence electrons in the chlorine atom or what is the valency of the chlorine, (because valency is the number of valence electrons present in the outermost valence shell).
Chlorine is having an atomic number of $17$ . Hence, the valency of the chlorine will be $7$ .Now, as chlorine has $7$ valence electrons, it makes $2$ double bonds with oxygen ( $2$ sigma and $2$ pi bonds), one with hydroxide ( $1$ sigma bond) leaving a lone pair on the chlorine atom.
So, the hybridisation of the compound will be $s{p^3}$ . So, the geometry of the compound will be tetrahedral. But due to the presence of a lone pair in the central atom the structure of the compound will be pyramidal as in the place of the one bond there is an lone pair, and we know that in the structure the lone pair is not shown. So, the final structure of the molecule would be pyramidal.
The structure is as shown in figure below:

Note:
When drawing any structure keep the points in mind that the valency of the central atom is complete and presence of lone pair take part while writing geometry, but while drawing the structure it would not be shown. Due to the presence of lone pairs, the geometry of the compound will be distorted.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




