
Draw the structure of the hexane molecule.
Answer
564.3k+ views
Hint:To answer this question, you must recall the rules for the IUPAC nomenclature of organic compounds which is given by the International Union of Pure and Applied Chemistry. The rules are given in such a way so as to make sure that each compound has a unique name.
Complete answer:
We are given the IUPAC name of the organic compound. First we take a look at the word root which gives us the number of carbon atoms present in the parent carbon chain. In other words it tells us the longest carbon chain in the compound. The word root in this compound is hex-. Thus, we can say that the longest carbon chain is of 6 carbon atoms.
Next we take a look at the suffix. The suffix represents the functional group present in the compound. In the given compound, the suffix is –al suggesting that the functional group present in the given compound is an aldehyde group.
In the name of the compound, we are not specified the position of the aldehyde group. In such cases we assume the specific position as 1. So we attach a carbonyl group on the first carbon of the parent carbon chain of the compound to get the required aldehyde.
The structure of hexanal molecule can be drawn as:
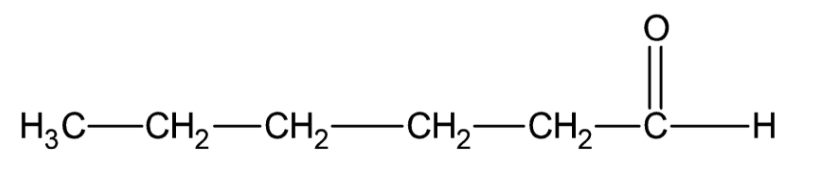
Note:
We are not given the position of the aldehyde group in the name of the compound, but we should know that this is the only possible structure of a compound having six carbon atoms with an aldehyde functional group.
Complete answer:
We are given the IUPAC name of the organic compound. First we take a look at the word root which gives us the number of carbon atoms present in the parent carbon chain. In other words it tells us the longest carbon chain in the compound. The word root in this compound is hex-. Thus, we can say that the longest carbon chain is of 6 carbon atoms.
Next we take a look at the suffix. The suffix represents the functional group present in the compound. In the given compound, the suffix is –al suggesting that the functional group present in the given compound is an aldehyde group.
In the name of the compound, we are not specified the position of the aldehyde group. In such cases we assume the specific position as 1. So we attach a carbonyl group on the first carbon of the parent carbon chain of the compound to get the required aldehyde.
The structure of hexanal molecule can be drawn as:
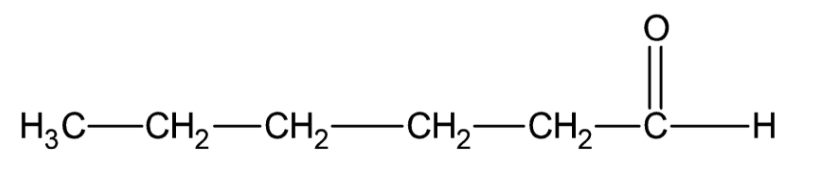
Note:
We are not given the position of the aldehyde group in the name of the compound, but we should know that this is the only possible structure of a compound having six carbon atoms with an aldehyde functional group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




