
Draw the structure of xenon tetrafluoride molecule and state the hybridization of the central atom and the geometry of the molecule.
Answer
572.1k+ views
Hint: First determine the steric number of the central xenon atom in xenon tetrafluoride. Then determine the hybridization and the geometry based on the steric number. Hybridisation is the most important factor to find out the geometry of any compound.
Complete Step by step answer: Xenon is a noble gas element with atomic number 54. Write the electronic configuration of xenon.
Xenon has completed its octet and is chemically unreactive. There are 8 valence electrons in the valence shell of the central xenon atom.
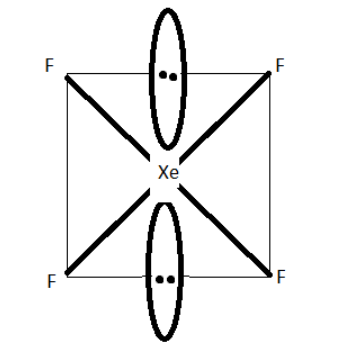
However, xenon forms few compounds with fluorine. Xenon tetrafluoride is one such compound. The central xenon atom in xenon tetrafluoride has four xenon-fluorine single covalent (sigma) bonds. To form these four bonds, a xenon atom shares 4 electrons with 4 electrons of 4 fluorine atoms. Thus, out of 8 valence electrons of xenon, 4 electrons are used in making 4 bonds and 4 electrons are remaining. These 4 electrons are present as two pairs of lone pairs (unshared pair or non-bonding pair) of electrons. A lone pair of electrons contains 2 electrons. So, 2 lone pairs of electrons contain 4 electrons.
Thus, the central xenon atom is surrounded by two lone pairs of electrons and 4 bond pairs of electrons. Thus, the central xenon atom is surrounded by a total six electron pairs.
You can say that the steric number of xenon atoms in xenon tetrafluoride is 6. The steric number of 6 is associated with \[s{p^3}{d^2}\] hybridization and the octahedral geometry. Thus, the central xenon atom in xenon tetrafluoride is \[s{p^3}{d^2}\] hybridized. Also, the electron pair geometry of xenon tetrafluoride molecule is octahedral and the molecular geometry is square planar.
Note: It should be noted that the Steric number is the total number of electron pairs around the central atom in a compound. The steric number includes a lone pair of electrons as well as a bond pair of electrons. It somehow represents how much hindrance is there around lone pairs of electrons.
Complete Step by step answer: Xenon is a noble gas element with atomic number 54. Write the electronic configuration of xenon.
Xenon has completed its octet and is chemically unreactive. There are 8 valence electrons in the valence shell of the central xenon atom.
However, xenon forms few compounds with fluorine. Xenon tetrafluoride is one such compound. The central xenon atom in xenon tetrafluoride has four xenon-fluorine single covalent (sigma) bonds. To form these four bonds, a xenon atom shares 4 electrons with 4 electrons of 4 fluorine atoms. Thus, out of 8 valence electrons of xenon, 4 electrons are used in making 4 bonds and 4 electrons are remaining. These 4 electrons are present as two pairs of lone pairs (unshared pair or non-bonding pair) of electrons. A lone pair of electrons contains 2 electrons. So, 2 lone pairs of electrons contain 4 electrons.
Thus, the central xenon atom is surrounded by two lone pairs of electrons and 4 bond pairs of electrons. Thus, the central xenon atom is surrounded by a total six electron pairs.
You can say that the steric number of xenon atoms in xenon tetrafluoride is 6. The steric number of 6 is associated with \[s{p^3}{d^2}\] hybridization and the octahedral geometry. Thus, the central xenon atom in xenon tetrafluoride is \[s{p^3}{d^2}\] hybridized. Also, the electron pair geometry of xenon tetrafluoride molecule is octahedral and the molecular geometry is square planar.

Note: It should be noted that the Steric number is the total number of electron pairs around the central atom in a compound. The steric number includes a lone pair of electrons as well as a bond pair of electrons. It somehow represents how much hindrance is there around lone pairs of electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




