
Draw the structures of the following: ${{H}_{2}}S{{O}_{3}}$ .
Answer
550.8k+ views
Hint: The given compound is sulphurous acid. Having a basic idea of bonds and the lone pairs of electrons will help us draw the structure of ${{H}_{2}}S{{O}_{3}}$.
Complete answer:
Let us have a quick look on the given compound and its properties before drawing the required structure;
Sulphurous acid-
This is also known as sulphur dioxide solution or dihydrogen tri oxo sulphate or tri oxo sulphuric acid. It is primarily the intermediate species while forming acid rain from sulphur dioxide i.e. $S{{O}_{2}}$ .
It is a colourless liquid and has a pungent smell when burned. It is used as a reducing agent, disinfectant and in the manufacturing of paper-based products.
Structure of ${{H}_{2}}S{{O}_{3}}$-
There are some rules while drawing the structure of any compound;
1. Hydrogen cannot be the central metal atom.
2. The hydrogen must be bonded with oxygen atom (if oxoacids are present).
3. The atom having lowest electronegativity value is usually the central metal atom.
Now, while drawing the structure for ${{H}_{2}}S{{O}_{3}}$ we will come across the presence of a lone pair of electrons on the sulphur atom. This will define the structure and its geometry; though oxygen too has two pairs each but will not affect the geometry. Thus, the structure of ${{H}_{2}}S{{O}_{3}}$ is,
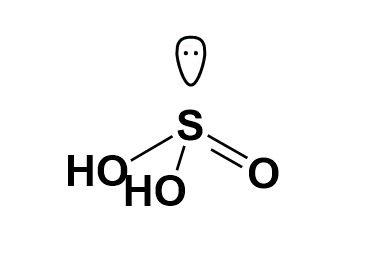
Note:
Sulphurous acid can be recognised by its suffocating smell and it is not highly corrosive as other acids which are strong as hydrochloric acid and sulphuric acid. Thus, this is the weak acid.
Complete answer:
Let us have a quick look on the given compound and its properties before drawing the required structure;
Sulphurous acid-
This is also known as sulphur dioxide solution or dihydrogen tri oxo sulphate or tri oxo sulphuric acid. It is primarily the intermediate species while forming acid rain from sulphur dioxide i.e. $S{{O}_{2}}$ .
It is a colourless liquid and has a pungent smell when burned. It is used as a reducing agent, disinfectant and in the manufacturing of paper-based products.
Structure of ${{H}_{2}}S{{O}_{3}}$-
There are some rules while drawing the structure of any compound;
1. Hydrogen cannot be the central metal atom.
2. The hydrogen must be bonded with oxygen atom (if oxoacids are present).
3. The atom having lowest electronegativity value is usually the central metal atom.
Now, while drawing the structure for ${{H}_{2}}S{{O}_{3}}$ we will come across the presence of a lone pair of electrons on the sulphur atom. This will define the structure and its geometry; though oxygen too has two pairs each but will not affect the geometry. Thus, the structure of ${{H}_{2}}S{{O}_{3}}$ is,

Note:
Sulphurous acid can be recognised by its suffocating smell and it is not highly corrosive as other acids which are strong as hydrochloric acid and sulphuric acid. Thus, this is the weak acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




