
How can I draw the structures of the two enantiomers of alanine?
Answer
558.6k+ views
Hint: Enantiomers are optically active compounds which are mirror images of one another. Alanine is a class of amino acid which belongs to α-amino acid.
Complete step by step answer:
The structure of a compound is the spatial arrangement of the groups of an organic molecule. The structure of a compound is expressed in two dimensions or in three dimensions. The arrangements of the groups in different positions in such a way so that the pairs of molecules are mirror images of one another are known as enantiomers.
Enantiomers are a type of stereoisomers. They contain the same atoms and molecules and only differ in the spatial arrangement of the atoms. A pair of enantiomers is non-superimposable mirror images of one another.
Alanine is an amino acid. Specifically it is an α-amino acid in which the amino group and the carboxylic acid group are attached to the same carbon atom. The chemical formula of alanine is written as $C{H_3}CH(N{H_2})COOH$.
The two structures of alanine are drawn in Fischer projection and 3-D structure as:
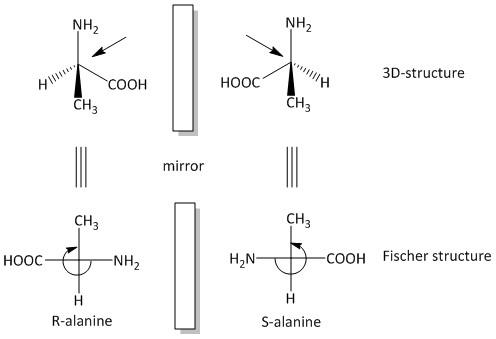
The structures on the above are 3-D structures of alanine and the structures on the below are 2-D Fischer structures. Both pairs of enantiomers are mirror images of one another. The clockwise rotation of the enantiomer on the left side is the \[R\]-isomer and the anticlockwise rotation of the enantiomer on the right side of the diagram is the \[S\]-isomer of alanine.
Note:
The clockwise and anticlockwise rotation of the enantiomers depends on the preference of the substituents attached to the chiral centre of the molecule. Thus the groups are labeled as a, b, c and so on. In moving from a to b to c if clockwise rotation is required then the molecule is \[R\]-isomer and if anticlockwise rotation is required then the molecule is \[S\]-isomer.
Complete step by step answer:
The structure of a compound is the spatial arrangement of the groups of an organic molecule. The structure of a compound is expressed in two dimensions or in three dimensions. The arrangements of the groups in different positions in such a way so that the pairs of molecules are mirror images of one another are known as enantiomers.
Enantiomers are a type of stereoisomers. They contain the same atoms and molecules and only differ in the spatial arrangement of the atoms. A pair of enantiomers is non-superimposable mirror images of one another.
Alanine is an amino acid. Specifically it is an α-amino acid in which the amino group and the carboxylic acid group are attached to the same carbon atom. The chemical formula of alanine is written as $C{H_3}CH(N{H_2})COOH$.
The two structures of alanine are drawn in Fischer projection and 3-D structure as:

The structures on the above are 3-D structures of alanine and the structures on the below are 2-D Fischer structures. Both pairs of enantiomers are mirror images of one another. The clockwise rotation of the enantiomer on the left side is the \[R\]-isomer and the anticlockwise rotation of the enantiomer on the right side of the diagram is the \[S\]-isomer of alanine.
Note:
The clockwise and anticlockwise rotation of the enantiomers depends on the preference of the substituents attached to the chiral centre of the molecule. Thus the groups are labeled as a, b, c and so on. In moving from a to b to c if clockwise rotation is required then the molecule is \[R\]-isomer and if anticlockwise rotation is required then the molecule is \[S\]-isomer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




