
During the preparation of phenol from benzene sulfonic acid, which of the following reagents is to be used?
(A) NaOH
(B) $NaHS{{O}_{4}}$
(C) $N{{a}_{2}}S{{O}_{4}}$
(D) $NaCl$
Answer
233.1k+ views
Hint: Benzene sulphonic acid which is a conjugate base of benzene sulphonate is an organosulfur compound with chemical formula ${{C}_{6}}{{H}_{6}}{{O}_{3}}S$and is the simplest aromatic sulfonic acid. It is a strong acid and dissociates fully in water.
Step by step answer:
-Benzene sulphonic acid is obtained by reacting benzene with oleum. The formed benzene sulphonic acid, when treated with molten sodium hydroxide at high temperature, favours the formation of sodium phenoxide ion which on further acidification gives phenol.
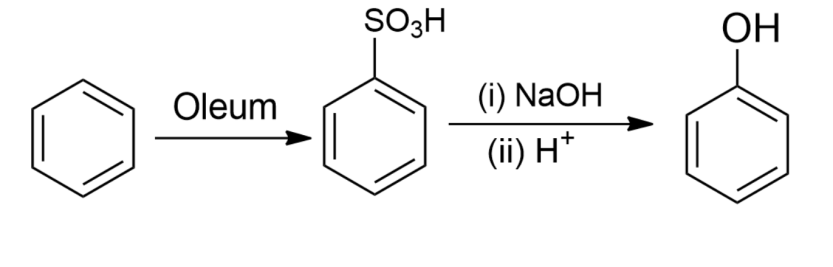
So, the correct answer is option A.
Additional information:
-Phenol can be prepared by many other processes which are described below-
(i) From haloarenes- Chlorobenzene which is formed by the monosubstitution of the benzene ring is an example of haloarene. When chlorobenzene is fused with sodium hydroxide at 626K and 20atm, sodium phenoxide is formed which on acidification gives phenol.
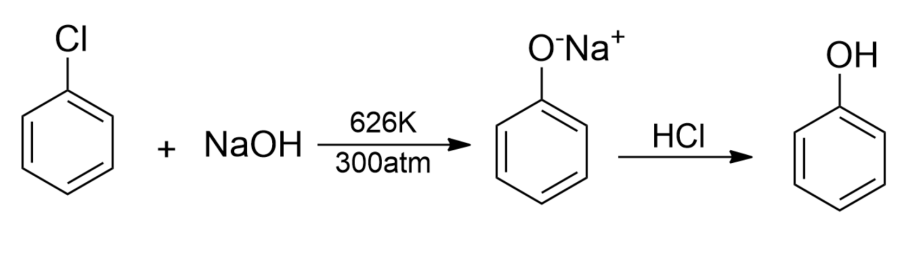
(ii) From Diazonium salts- Diazonium salts are obtained when an aromatic primary amine is treated with nitrous acid $(NaN{{O}_{2}}+HCl)$at about 273-278K. These diazonium salts are chemically highly reactive. The diazonium salts upon warming with water hydrolyzes to phenols.

(iii) From cumene- Cumene is an organic compound produced by Friedel Crafts alkylation of benzene with propylene. Oxidation of cumene in the presence of air gives hydroperoxide which on further treatment with dilute acids gives phenol along with acetone as side by-products in large quantities. Hence phenols formed by this method need purifications.
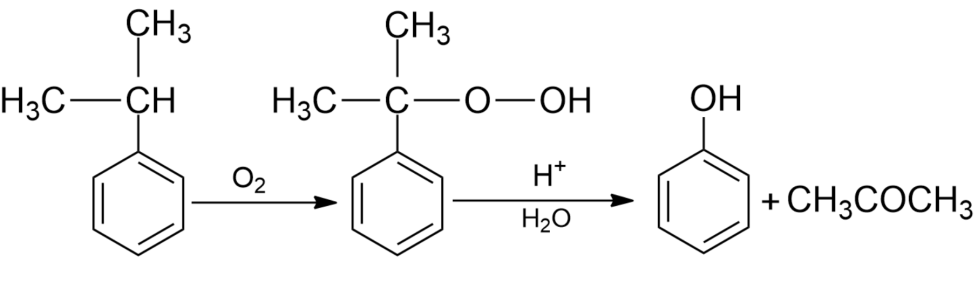
Note: The alkali metal salt of benzene sulphonic acid used in the industrial production of phenol involves the presence of phenoxide salt and produced a lot of water and side products. This process is sometimes known as alkaline fusion. Now, this process is replaced by the Hock process for the production of phenol as it produces less waste.
Step by step answer:
-Benzene sulphonic acid is obtained by reacting benzene with oleum. The formed benzene sulphonic acid, when treated with molten sodium hydroxide at high temperature, favours the formation of sodium phenoxide ion which on further acidification gives phenol.

So, the correct answer is option A.
Additional information:
-Phenol can be prepared by many other processes which are described below-
(i) From haloarenes- Chlorobenzene which is formed by the monosubstitution of the benzene ring is an example of haloarene. When chlorobenzene is fused with sodium hydroxide at 626K and 20atm, sodium phenoxide is formed which on acidification gives phenol.

(ii) From Diazonium salts- Diazonium salts are obtained when an aromatic primary amine is treated with nitrous acid $(NaN{{O}_{2}}+HCl)$at about 273-278K. These diazonium salts are chemically highly reactive. The diazonium salts upon warming with water hydrolyzes to phenols.

(iii) From cumene- Cumene is an organic compound produced by Friedel Crafts alkylation of benzene with propylene. Oxidation of cumene in the presence of air gives hydroperoxide which on further treatment with dilute acids gives phenol along with acetone as side by-products in large quantities. Hence phenols formed by this method need purifications.

Note: The alkali metal salt of benzene sulphonic acid used in the industrial production of phenol involves the presence of phenoxide salt and produced a lot of water and side products. This process is sometimes known as alkaline fusion. Now, this process is replaced by the Hock process for the production of phenol as it produces less waste.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




