
$EDT{{A}^{4-}}$ is ethylene diamine tetraacetate ion. The total number of $-N-Co-O$ bond angles in ${{[Co(EDTA)]}^{-}}$ complex ion is:
Answer
579.9k+ views
Hint: To get the total number of bond angles, first try to draw the chemical structure of the ethylene diamine tetraacetate ion and then see what structure it gives when it is treated with cobalt atoms. Draw the chemical structure of the complex formed, then you will get an idea about the number of bond angles present in the complex.
Complete step by step answer:
So, before proceeding to find the answer, let us know about ethylene diamine tetraacetate, in short referred to as EDTA.
So, EDTA is a hexadentate ligand, which implies that it binds multiple (six) times. It binds twice at the nitrogen and four at the oxygen atoms. It has an atomic structure a lot like a claw. The chemical structure of ethylene diamine tetraacetate ion i.e. $EDT{{A}^{4-}}$ is shown in the figure below:
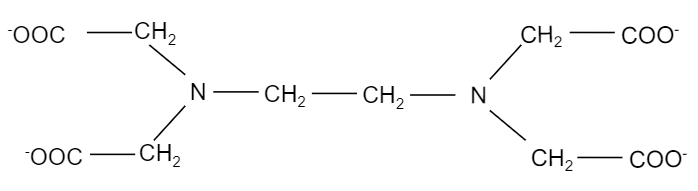
When ethylene diamine tetraacetate ion is treated with cobalt element, it forms a complex, ${{[Co(EDTA)]}^{-}}$ where, cobalt binds with two nitrogen atoms and four oxygen ions present in the EDTA structure. The structure of the complex is shown below:
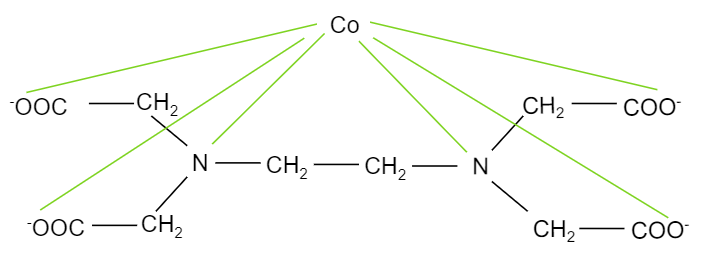
The oxygen atoms having negative charges and the two nitrogen atoms will be donating electrons forming bonds with cobalt. So, we can see that, in the complex, each nitrogen has four $-N-Co-O$ bond angles. And there are two nitrogen atoms bonded with cobalt. So, the total number of $-N-Co-O$ bond angles will be eight.
Hence, the total number of $-N-Co-O$ bond angles in ${{[Co(EDTA)]}^{-}}$ complex ion is eight.
Note: Metal analysis can be done by using the process of titration of the ethylene diamine tetraacetate and a metal where metal ion acts as the indicator. One of the most famous and most common uses of EDTA is to determine the hardness of the water.
Complete step by step answer:
So, before proceeding to find the answer, let us know about ethylene diamine tetraacetate, in short referred to as EDTA.
So, EDTA is a hexadentate ligand, which implies that it binds multiple (six) times. It binds twice at the nitrogen and four at the oxygen atoms. It has an atomic structure a lot like a claw. The chemical structure of ethylene diamine tetraacetate ion i.e. $EDT{{A}^{4-}}$ is shown in the figure below:
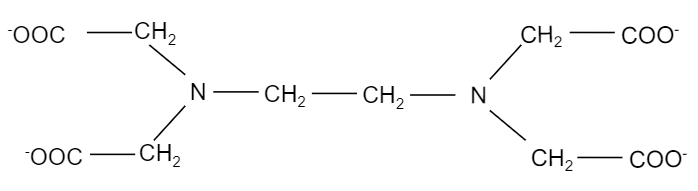
When ethylene diamine tetraacetate ion is treated with cobalt element, it forms a complex, ${{[Co(EDTA)]}^{-}}$ where, cobalt binds with two nitrogen atoms and four oxygen ions present in the EDTA structure. The structure of the complex is shown below:

The oxygen atoms having negative charges and the two nitrogen atoms will be donating electrons forming bonds with cobalt. So, we can see that, in the complex, each nitrogen has four $-N-Co-O$ bond angles. And there are two nitrogen atoms bonded with cobalt. So, the total number of $-N-Co-O$ bond angles will be eight.
Hence, the total number of $-N-Co-O$ bond angles in ${{[Co(EDTA)]}^{-}}$ complex ion is eight.
Note: Metal analysis can be done by using the process of titration of the ethylene diamine tetraacetate and a metal where metal ion acts as the indicator. One of the most famous and most common uses of EDTA is to determine the hardness of the water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




