
What is the electron configuration for phosphide ions?
Answer
492.9k+ views
Hint: To obtain the electron configuration of an ion, we first compute the electron configuration of the neutral atom, then change the number of electrons in the configuration to produce an octet based on the number of electrons gained or lost.
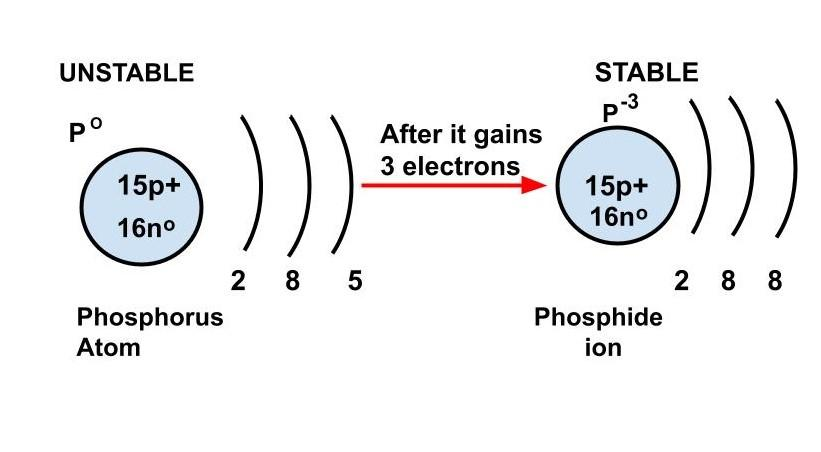
Complete answer: Phosphide is a chemical compound in which phosphorus has been bonded with a metal. \[{P^{3 - }}\] is the phosphide ion, and there are phosphides of nearly every metal in the periodic table. They have a wide range of chemical and physical characteristics.
The electron configuration of phosphorus is $\left[ {Ne} \right]3{s^2}3{p^3}$
The phosphorus atom gains three electrons when the phosphide ion forms.
As a result, the electron configuration changes $\left[ {Ne} \right]3{s^2}3{p^6}$ because three electrons are contributed to the \[3p\] subshell by the phosphorus atom
Additional information:
Although phosphides can be made in a variety of ways, the most common is to heat stoichiometric proportions of metal and red phosphorus to high temperatures in an inert atmosphere (one devoid of chemically reactive compounds) or in a vacuum. Electrolysis reactions, the reaction of a metal (or a metal halide or metal sulphide) with phosphine \[\left( {P{H_3}} \right)\] , and the reduction of a metal phosphate with elemental carbon at an increased temperature are all ways that can be used.
Note:
The phosphides of electropositive alkali metals and alkaline-earth metals display ionic bonding that is extremely near to it. When these chemicals come into contact with water or a weak acid, they form phosphine, or \[P{H_3}\] .
Complete answer: Phosphide is a chemical compound in which phosphorus has been bonded with a metal. \[{P^{3 - }}\] is the phosphide ion, and there are phosphides of nearly every metal in the periodic table. They have a wide range of chemical and physical characteristics.
The electron configuration of phosphorus is $\left[ {Ne} \right]3{s^2}3{p^3}$
The phosphorus atom gains three electrons when the phosphide ion forms.
As a result, the electron configuration changes $\left[ {Ne} \right]3{s^2}3{p^6}$ because three electrons are contributed to the \[3p\] subshell by the phosphorus atom

Additional information:
Although phosphides can be made in a variety of ways, the most common is to heat stoichiometric proportions of metal and red phosphorus to high temperatures in an inert atmosphere (one devoid of chemically reactive compounds) or in a vacuum. Electrolysis reactions, the reaction of a metal (or a metal halide or metal sulphide) with phosphine \[\left( {P{H_3}} \right)\] , and the reduction of a metal phosphate with elemental carbon at an increased temperature are all ways that can be used.
Note:
The phosphides of electropositive alkali metals and alkaline-earth metals display ionic bonding that is extremely near to it. When these chemicals come into contact with water or a weak acid, they form phosphine, or \[P{H_3}\] .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




