
What is the electron configuration, orbital diagram, and noble gas notation of potassium?
Answer
531.3k+ views
Hint :We know that the atomic number of potassium is 19. The electronic configuration of an element describes how electrons are distributed in its atomic orbital. Using the electronic configuration we can draw the orbital diagram and noble gas notation.
Complete Step By Step Answer:
We know that the atomic number of potassium is 19. That is every atom of potassium has 19 protons in its nucleus. In a neutral atom, the number of protons is equal to the number of electrons. So the electronic configuration of potassium will involve 19 electrons and it is given by,
$ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1} $
The noble gas notation of an atom consists of the last Noble gas prior to that atom, followed by the configuration of the remaining electrons.
Then we will have $ [Ar]4{s^1} $
The following orbital diagram shows the increase in energy from one energy sublevel to the next but we can write them on the same level horizontally.
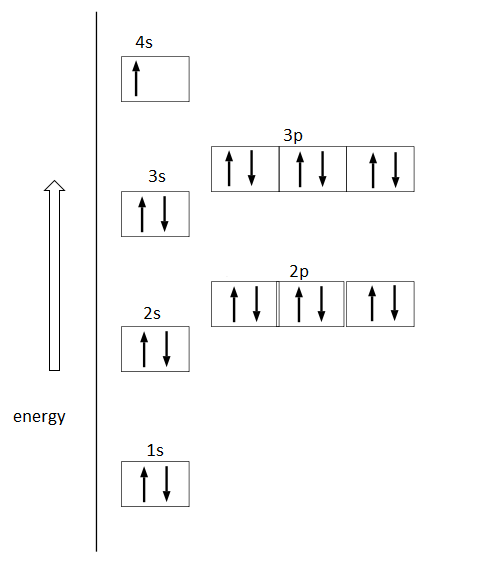
Note :
Orbital diagrams are pictorial descriptions of the electrons in an atom. Potassium is one of the most important minerals in the body. It helps regulate fluid balance, muscle contractions and nerve signals. High potassium diet may help reduce blood pressure and water retention.
Complete Step By Step Answer:
We know that the atomic number of potassium is 19. That is every atom of potassium has 19 protons in its nucleus. In a neutral atom, the number of protons is equal to the number of electrons. So the electronic configuration of potassium will involve 19 electrons and it is given by,
$ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^1} $
The noble gas notation of an atom consists of the last Noble gas prior to that atom, followed by the configuration of the remaining electrons.
Then we will have $ [Ar]4{s^1} $
The following orbital diagram shows the increase in energy from one energy sublevel to the next but we can write them on the same level horizontally.

Note :
Orbital diagrams are pictorial descriptions of the electrons in an atom. Potassium is one of the most important minerals in the body. It helps regulate fluid balance, muscle contractions and nerve signals. High potassium diet may help reduce blood pressure and water retention.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




