
What is the electron dot diagram for zinc?
Answer
524.1k+ views
Hint: Electron dot diagram also known as Lewis diagram is a way to represent the number of electrons present in the valence shell of an element. In the diagram, the number of dots on the symbol of the element represent its valence electrons.
Complete answer:
To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau’s Principle.
Aufbau’s Principle: It states that the electrons must be filled first in orbitals with lower energy than the orbitals which have comparatively higher energy.
Atomic number of Zinc $ = 30$
Electronic configuration $ = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}$
The energy of $4s$ shell is less as compared to $3d$ shell and hence according to Aufbau Principle, the electrons are first filled in $4s$ shell. But the $3d$ shell is inner shell orbital, therefore electrons present in $4s$ orbital are considered valence shell electrons.
Hence the electron dot diagram or Lewis diagram of Zinc is represented as follows:
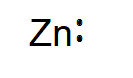
Additional Information:
Zinc is a metal present in the first transition series. Its atomic number is $30$ . Although it is present in $3d$ series , it is not considered as a transition metal because of its completely filled d-orbital. It shows variable oxidation states and it is a strong reducing agent.
Note:
Electrons present in the outer shell orbitals are only considered as valence shell electrons. The electronic configuration of the element should follow Aufbau’s Principle and Hund’s rule and representation of valence electrons is done by representing dots on the structure.
Complete answer:
To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau’s Principle.
Aufbau’s Principle: It states that the electrons must be filled first in orbitals with lower energy than the orbitals which have comparatively higher energy.
Atomic number of Zinc $ = 30$
Electronic configuration $ = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}$
The energy of $4s$ shell is less as compared to $3d$ shell and hence according to Aufbau Principle, the electrons are first filled in $4s$ shell. But the $3d$ shell is inner shell orbital, therefore electrons present in $4s$ orbital are considered valence shell electrons.
Hence the electron dot diagram or Lewis diagram of Zinc is represented as follows:
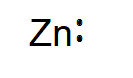
Additional Information:
Zinc is a metal present in the first transition series. Its atomic number is $30$ . Although it is present in $3d$ series , it is not considered as a transition metal because of its completely filled d-orbital. It shows variable oxidation states and it is a strong reducing agent.
Note:
Electrons present in the outer shell orbitals are only considered as valence shell electrons. The electronic configuration of the element should follow Aufbau’s Principle and Hund’s rule and representation of valence electrons is done by representing dots on the structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




