
What is the electron dot structure for $PC{{l}_{3}}$?
Answer
523.8k+ views
Hint: Electron dot structures or diagrams are also called Lewis structure. They are the representation of the total valence electrons in any atom or molecule. For polyatomic species, the valence electrons of each atom are calculated and added, then arranged on the individual atoms to obtain an electron dot structure. The dots represent electrons.
Complete answer:
The electrons dot structures or Lewis dot diagrams is the representation of the atoms in the molecules having electrons in the valence shell drawn as dots around the atom. There are certain rules to draw electron dot structures that are. We have been given$PC{{l}_{3}}$that is phosphorus trichloride. The rules for making its electron dot structure are:
-The total number of valence electrons is calculated in the atoms and is added. In$PC{{l}_{3}}$, P has 5 and 3 chlorine atoms and 21 electrons in valence shell that are a total = 26 electrons.
- The most electronegative element occupies the central position in the structure. Here phosphorus is more electronegative, so it is placed in the middle.
- The total number of valence electrons is then distributed to form bond pairs and lone pair of electrons. Two electrons that are shared between atoms form a bond.
- The remaining electrons are then placed over the atoms, so that they have a complete octet. Those electrons that do not complete octet are placed in sharing with the atoms and thus forming bonds.
Therefore, the electron dot structure of $PC{{l}_{3}}$according to the rules is made to be as
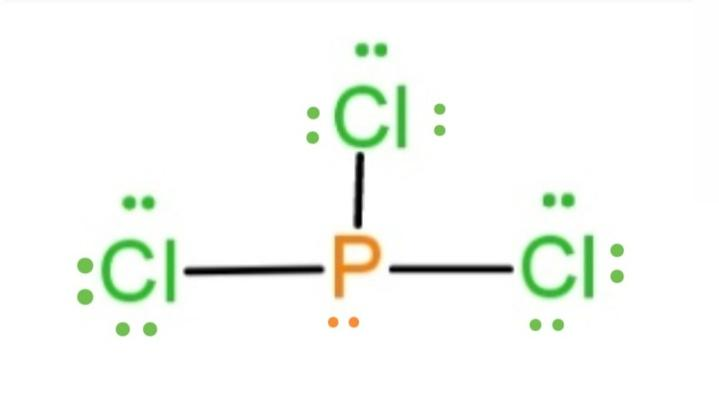
Hence, the above dot structure shows the valence electrons as dots placed on the atoms in $PC{{l}_{3}}$.
Note:
The octet rule implies here that states that each atom is having 8 electrons in its valence shell to complete the octet and hence acquire a fully filled shell that makes a molecule stable. The places of lone pair and bond pair of electrons on the atom leads to the geometry of the molecule and thus to the shape of the molecule. Due to the lone pair of electrons on phosphorus $PC{{l}_{3}}$ has a trigonal bi pyramidal shape.
Complete answer:
The electrons dot structures or Lewis dot diagrams is the representation of the atoms in the molecules having electrons in the valence shell drawn as dots around the atom. There are certain rules to draw electron dot structures that are. We have been given$PC{{l}_{3}}$that is phosphorus trichloride. The rules for making its electron dot structure are:
-The total number of valence electrons is calculated in the atoms and is added. In$PC{{l}_{3}}$, P has 5 and 3 chlorine atoms and 21 electrons in valence shell that are a total = 26 electrons.
- The most electronegative element occupies the central position in the structure. Here phosphorus is more electronegative, so it is placed in the middle.
- The total number of valence electrons is then distributed to form bond pairs and lone pair of electrons. Two electrons that are shared between atoms form a bond.
- The remaining electrons are then placed over the atoms, so that they have a complete octet. Those electrons that do not complete octet are placed in sharing with the atoms and thus forming bonds.
Therefore, the electron dot structure of $PC{{l}_{3}}$according to the rules is made to be as

Hence, the above dot structure shows the valence electrons as dots placed on the atoms in $PC{{l}_{3}}$.
Note:
The octet rule implies here that states that each atom is having 8 electrons in its valence shell to complete the octet and hence acquire a fully filled shell that makes a molecule stable. The places of lone pair and bond pair of electrons on the atom leads to the geometry of the molecule and thus to the shape of the molecule. Due to the lone pair of electrons on phosphorus $PC{{l}_{3}}$ has a trigonal bi pyramidal shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




