
How many electrons in an atom may have the following quantum numbers?
a.) n = 4, m$_s$ = -1/2
b.) n = 3, l = 0
Answer
588.6k+ views
Hint: We know that there are a fixed number of electrons in each shell, and the electrons attained in a fixed number are multiples of 2. This question is based on the formula of total number of electrons in terms of n.
Complete step by step answer:
-First, let us know about some terms related to the electron shell. The first is the principal quantum number represented by n, and its value is n = 1, 2, 3, 4, and so on.
-The second is orbital angular momentum represented by L, if we have L = 0 it means it has s orbital, L=1 then p- orbital, and so on.
-The third is spin quantum number m$_s$ that exists as +1/2 or -1/2, it means that an electron can spin in only two directions up, and down. +1/2 for up, and -1/2 for downside.
-We know that total number of electrons in n shell = 2n$^{2}$ (Formula)
-Now, for (a) part, we are given n = 4, by applying the formula,
Total number of electrons in n = 4 shell will be 32
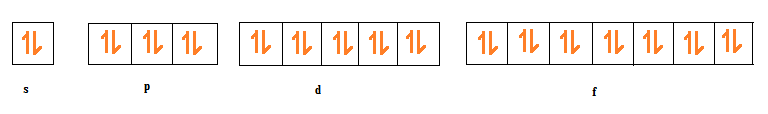
But, we are also given the spin quantum number i.e.-1/2, it means we have to consider the electrons with the negative spin only.
Therefore, the total number of electrons for n= 4 shells having -1/2 spin will be 16.
-Now, for (b) part, we are given n=3, l=0
As mentioned, l = 0 means s orbital, so it is a 3s orbital.
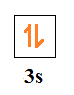
Then, the number of electrons in 3s orbital is 2.
-In the last, we can conclude that (a) part number of electrons is 16, and (b) part number of electrons are 2.
Note: Don’t get confused while finding the number of electrons. Look at the given terms in the question, and then find. There can be confusion as to why we halved the number of electrons for negative spin, then we did it because the number of electrons for positive and negative spin is equal.
Complete step by step answer:
-First, let us know about some terms related to the electron shell. The first is the principal quantum number represented by n, and its value is n = 1, 2, 3, 4, and so on.
-The second is orbital angular momentum represented by L, if we have L = 0 it means it has s orbital, L=1 then p- orbital, and so on.
-The third is spin quantum number m$_s$ that exists as +1/2 or -1/2, it means that an electron can spin in only two directions up, and down. +1/2 for up, and -1/2 for downside.
-We know that total number of electrons in n shell = 2n$^{2}$ (Formula)
-Now, for (a) part, we are given n = 4, by applying the formula,
Total number of electrons in n = 4 shell will be 32

But, we are also given the spin quantum number i.e.-1/2, it means we have to consider the electrons with the negative spin only.
Therefore, the total number of electrons for n= 4 shells having -1/2 spin will be 16.
-Now, for (b) part, we are given n=3, l=0
As mentioned, l = 0 means s orbital, so it is a 3s orbital.

Then, the number of electrons in 3s orbital is 2.
-In the last, we can conclude that (a) part number of electrons is 16, and (b) part number of electrons are 2.
Note: Don’t get confused while finding the number of electrons. Look at the given terms in the question, and then find. There can be confusion as to why we halved the number of electrons for negative spin, then we did it because the number of electrons for positive and negative spin is equal.
Watch videos on
How many electrons in an atom may have the following quantum numbers?
a.) n = 4, m$_s$ = -1/2
b.) n = 3, l = 0
a.) n = 4, m$_s$ = -1/2
b.) n = 3, l = 0

Structure of atom class 11 Chemistry -NCERT EXERCISE 2.31 | Chemistry | Sumandeep Ma'am
Subscribe
 Share
Share likes
6.4K Views
2 years ago
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE



 Watch Video
Watch Video
