
Electrons left in the valence shell when Xe is converted to $Xe{F_6}$ is ___.
Answer
571.8k+ views
Hint:The chemical element xenon is represented by Xe. The atomic number of xenon is 54. The electronic configuration of xenon is $[Kr]4{d^{10}}5{s^2}5{p^6}$. It is a noble gas which has a stable configuration as it fulfills the octet rule which says that for an atom to be stable it should possess 8 electrons in its valence shell.
Complete answer:The element Xenon (Xe) forms xenon hexafluoride in which one xenon atom is bonded with six fluorine atoms by a sigma bond.
The chemical bond is formed by sharing of electrons by the atoms to form a compound. To form a sigma bond each atom shares one electron each to form a molecule.
The atomic number of xenon is 54. The electronic configuration of xenon is $[Kr]4{d^{10}}5{s^2}5{p^6}$. It has 8 valence electrons in its outermost electronic configuration so it can share its 8 electrons with other atoms.
As in xenon hexafluoride xenon is bonded with six fluorine atoms. It donates its six electrons to six fluorine atoms to form six bonds and two electrons are left in the valence shell.
The structure of Xenon hexafluoride is shown below.
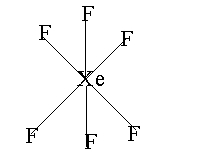
Note: The two electrons which do not take part in forming bonds are called non-bonding electrons. These two electrons are present as the lone pair above the central atom. The hybridization of xenon hexafluoride is $s{p^3}{d^3}$ and the geometry is distorted octahedral. The bond angle is 90 deg and 72 deg.
Complete answer:The element Xenon (Xe) forms xenon hexafluoride in which one xenon atom is bonded with six fluorine atoms by a sigma bond.
The chemical bond is formed by sharing of electrons by the atoms to form a compound. To form a sigma bond each atom shares one electron each to form a molecule.
The atomic number of xenon is 54. The electronic configuration of xenon is $[Kr]4{d^{10}}5{s^2}5{p^6}$. It has 8 valence electrons in its outermost electronic configuration so it can share its 8 electrons with other atoms.
As in xenon hexafluoride xenon is bonded with six fluorine atoms. It donates its six electrons to six fluorine atoms to form six bonds and two electrons are left in the valence shell.
The structure of Xenon hexafluoride is shown below.

Note: The two electrons which do not take part in forming bonds are called non-bonding electrons. These two electrons are present as the lone pair above the central atom. The hybridization of xenon hexafluoride is $s{p^3}{d^3}$ and the geometry is distorted octahedral. The bond angle is 90 deg and 72 deg.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




