
Energy of the activated complex is represented by:
A.A
B.B
C.C
D.D

Answer
568.5k+ views
Hint: The Activation energy is the minimum amount of energy that is required to transform reactants into products in a chemical reaction and the activated complex has the highest energy state. The energy of the activated complex is measured from the very bottom of the diagram to the top of the activation energy barrier.
Complete step by step answer:
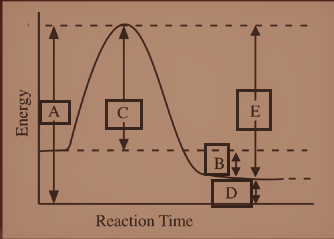
The potential energy diagram above gives the relative energy of the reactants and the products in a reaction. The x-axis represents the pathway of the reaction, so in this general case, we have:
reactants $ \to $ products
The particles that are the reactants in this reaction have a certain amount of potential energy (y-axis).
To start a reaction, the reactants must have a certain amount of energy, otherwise, the products cannot be formed. The activation energy thus can be seen as a barrier that must be overcome by reactants before it proceeds and the products can be formed. In the diagram, we can find this back as the 'hill' between the reactants and the products. In this picture, the hill is "Activated complex".
When the reactants overcome this barrier, the products are formed and to overcome this barrier, the reactants need some energy, which is called the activation energy of a reaction. We know, the activated complex has the highest energy state. The energy of the activated complex is the same as the height of the hill i.e. from the very bottom of the diagram to the top of the activation energy barrier and is thus displayed with ‘A’.
Thus, the energy of the activated complex is represented by "A"
Therefore, the correct answer is option (A).
Note: The activated complex or the transition complex is the complex that exists as the bonds in the products are forming and the bonds in the reactants are breaking. This complex exists for a very short period of time and is found when the energy of the system is at its maximum.
Complete step by step answer:
The potential energy diagram above gives the relative energy of the reactants and the products in a reaction. The x-axis represents the pathway of the reaction, so in this general case, we have:
reactants $ \to $ products
The particles that are the reactants in this reaction have a certain amount of potential energy (y-axis).
To start a reaction, the reactants must have a certain amount of energy, otherwise, the products cannot be formed. The activation energy thus can be seen as a barrier that must be overcome by reactants before it proceeds and the products can be formed. In the diagram, we can find this back as the 'hill' between the reactants and the products. In this picture, the hill is "Activated complex".
When the reactants overcome this barrier, the products are formed and to overcome this barrier, the reactants need some energy, which is called the activation energy of a reaction. We know, the activated complex has the highest energy state. The energy of the activated complex is the same as the height of the hill i.e. from the very bottom of the diagram to the top of the activation energy barrier and is thus displayed with ‘A’.
Thus, the energy of the activated complex is represented by "A"
Therefore, the correct answer is option (A).
Note: The activated complex or the transition complex is the complex that exists as the bonds in the products are forming and the bonds in the reactants are breaking. This complex exists for a very short period of time and is found when the energy of the system is at its maximum.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




