
Equivalent mass of sugar is:
(A) $\dfrac{M}{{11}}$
(B) $\dfrac{M}{{12}}$
(C) $\dfrac{M}{{22}}$
(D) None of these
Answer
588k+ views
Hint: Equivalent mass or gram equivalent is mass if one equivalent which will combine with another substance in a chemical reaction the equivalent mass of a compound can be calculated by cajoling the molecular mass of compound with number of electrons gained or lost.
Complete step by step answer:
Sugar is a carbohydrate with a churl call formula ${C_{12}}{H_{22}}{O_{11}}$ It is also known as serosa cane or beet root.
Equivalent mass of sugar can be calculated by using the formula:-
Equivalent mass =$\dfrac{{Molecular Mass}}{{number of electron}}$ gained or lost.
Sucrose is a disaccharide of glucose and fructose its structure is
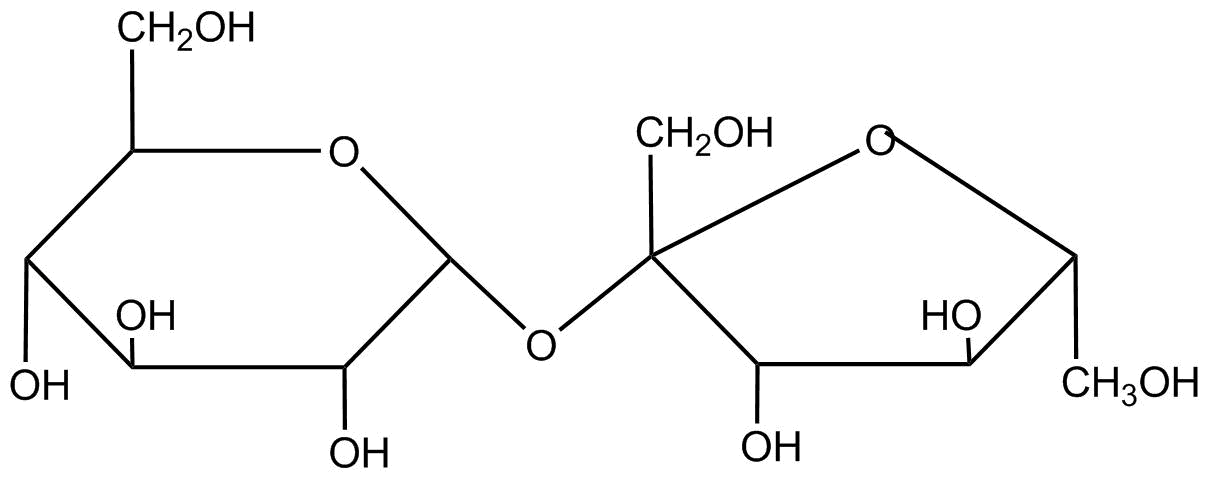
When sucrose undergoes a carnival reaction it gets reduced in it as loss of hydrogen’s present in it. As according to its formula${C_{12}}{H_{22}}{O_{11}}$, sucrose has 22 hydrogen atoms. Therefore, it loses 22 hydro-gent atoms.
Therefore equivalent mass of sucrose is:-
Equivalent mass =$\dfrac{{Molecular Mass}}{{22}}$=$\dfrac{M}{{22}}$
Note:- the actual equivalent mass of sugar is equivalent mass =$\dfrac{{molecular mass}}{{number of electrons}}$gained or lost.
Molecular mass of ${C_{12}}{H_{22}}{O_{11}} = 12(12) + 22(1) + 11(16) = 342G$
Equivalent mass $\dfrac{{342}}{{22}} = 15.54g$
Hence the correct option is C.
Note:
There are various types of Sugar
Fructose: found in fruits and honey.
Galactose: found in milk and dairy products.
Glucose: found in honey, fruits and vegetables.
Lactose: found in milk, made from glucose and galactose.
Maltose: found in barley.
Sucrose: made up of glucose and fructose and found in plants.
Xylose: found in wood or straw.
Complete step by step answer:
Sugar is a carbohydrate with a churl call formula ${C_{12}}{H_{22}}{O_{11}}$ It is also known as serosa cane or beet root.
Equivalent mass of sugar can be calculated by using the formula:-
Equivalent mass =$\dfrac{{Molecular Mass}}{{number of electron}}$ gained or lost.
Sucrose is a disaccharide of glucose and fructose its structure is

When sucrose undergoes a carnival reaction it gets reduced in it as loss of hydrogen’s present in it. As according to its formula${C_{12}}{H_{22}}{O_{11}}$, sucrose has 22 hydrogen atoms. Therefore, it loses 22 hydro-gent atoms.
Therefore equivalent mass of sucrose is:-
Equivalent mass =$\dfrac{{Molecular Mass}}{{22}}$=$\dfrac{M}{{22}}$
Note:- the actual equivalent mass of sugar is equivalent mass =$\dfrac{{molecular mass}}{{number of electrons}}$gained or lost.
Molecular mass of ${C_{12}}{H_{22}}{O_{11}} = 12(12) + 22(1) + 11(16) = 342G$
Equivalent mass $\dfrac{{342}}{{22}} = 15.54g$
Hence the correct option is C.
Note:
There are various types of Sugar
Fructose: found in fruits and honey.
Galactose: found in milk and dairy products.
Glucose: found in honey, fruits and vegetables.
Lactose: found in milk, made from glucose and galactose.
Maltose: found in barley.
Sucrose: made up of glucose and fructose and found in plants.
Xylose: found in wood or straw.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




