
Equivalent weight of pyrophosphoric acid is \[\left( {{H_4}{P_2}{O_7}} \right)\] is:
\[(i){\text{ }}\dfrac{{molecular{\text{ }}weight}}{1}\]
\[(ii){\text{ }}\dfrac{{molecular{\text{ }}weight}}{2}\]
\[(iii){\text{ }}\dfrac{{molecular{\text{ }}weight}}{4}\]
\[(iv){\text{ }}\dfrac{{molecular{\text{ }}weight}}{3}\]
Answer
493.2k+ views
Hint: Equivalent weight is the ratio of molecular weight and \[n - \]factor. The \[n - \]factor can be equal to its basicity or acidity depending on the compound. Pyrophosphoric is basic in nature. Therefore we will calculate the basicity of the pyrophosphoric acid and then divide the molecular weight by its basicity. This will give us its equivalent weight.
Complete answer:
The equivalent weight of a compound is the ratio of its molecular weight and \[n - \]factor. For acid and base this \[n - \]factor is basically its acidity and basicity. Basicity of a compound may be defined as the number of hydroxyl groups present in the given compound. Also it can be defined as the number of basic units which is attached to the given compound. We are given pyrophosphoric acid. We will find the basicity of pyrophosphoric acid by looking at the structure. The structure of pyrophosphoric acid can be drawn as,
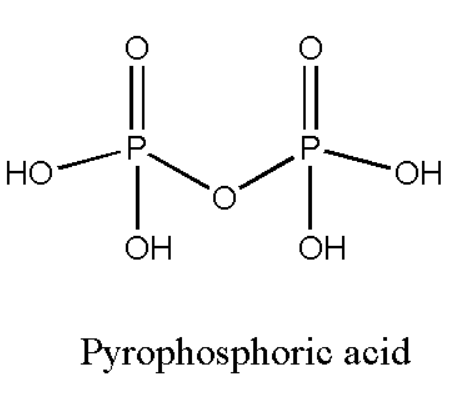
We can observe that the compound has four hydroxyl groups which will act as the basic unit for the given compound. Hence its basicity is four. According to definition of equivalent weight we can calculate its equivalent weight as,
Equivalent weight \[{\text{ = }}\dfrac{{molecular{\text{ }}weight}}{4}\]
Therefore the correct option is \[(iii){\text{ }}\dfrac{{molecular{\text{ }}weight}}{4}\].
Note:
For acids, we will calculate the acidity same as we calculate basicity of pyrophosphoric acid. We cannot find the basicity or acidity by knowing the molecular formula. We must draw the structural formula for finding the exact basicity and acidity of the given compound. If we are given the number of hydrogen loss or gain then, it is also called an acidity or basicity of the given compound.
Complete answer:
The equivalent weight of a compound is the ratio of its molecular weight and \[n - \]factor. For acid and base this \[n - \]factor is basically its acidity and basicity. Basicity of a compound may be defined as the number of hydroxyl groups present in the given compound. Also it can be defined as the number of basic units which is attached to the given compound. We are given pyrophosphoric acid. We will find the basicity of pyrophosphoric acid by looking at the structure. The structure of pyrophosphoric acid can be drawn as,

We can observe that the compound has four hydroxyl groups which will act as the basic unit for the given compound. Hence its basicity is four. According to definition of equivalent weight we can calculate its equivalent weight as,
Equivalent weight \[{\text{ = }}\dfrac{{molecular{\text{ }}weight}}{4}\]
Therefore the correct option is \[(iii){\text{ }}\dfrac{{molecular{\text{ }}weight}}{4}\].
Note:
For acids, we will calculate the acidity same as we calculate basicity of pyrophosphoric acid. We cannot find the basicity or acidity by knowing the molecular formula. We must draw the structural formula for finding the exact basicity and acidity of the given compound. If we are given the number of hydrogen loss or gain then, it is also called an acidity or basicity of the given compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




