
Ethyl alcohol is more soluble in water than dimethyl ether because:
A) Ether is a nonpolar molecule.
B) Alcohol contains hydrogen bonding.
C) Ether contains hydrogen bonding.
D) In ether, oxygen atoms are connected with water.
Answer
581.4k+ views
Hint: The bonding between an electronegative atom and a hydrogen atom bonded to another electronegative atom like oxygen, nitrogen or fluorine is known as hydrogen bonding. When the bonded atoms have a difference in their electronegativities the molecule is a polar molecule. When the bonded atoms do not differ in their electronegativities the molecule is nonpolar.
Complete step by step answer:
The structures of ethyl alcohol and dimethyl ether is as follows:
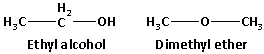
From the structures of ethyl alcohol and dimethyl ether, we can say that there is electronegativity difference in the bonded atoms. Thus, both ethyl alcohol and dimethyl ether are polar molecules. Water is also polar in nature. According to the rule ‘like dissolves like’, ethyl alcohol and dimethyl ether which are polar in nature are soluble in water which is also polar.
Now, we have to find out which amongst the two is more soluble in water. For this, we take into account the hydrogen bonding. The bonding between an electronegative atom and a hydrogen atom bonded to another electronegative atom like oxygen, nitrogen or fluorine is known as hydrogen bonding.
In ethyl alcohol, the hydrogen atom is bonded to an electronegative oxygen atom. Thus, hydrogen bonding is possible in ethyl alcohol. The hydrogen bonding in ethyl alcohol is shown in the following image.
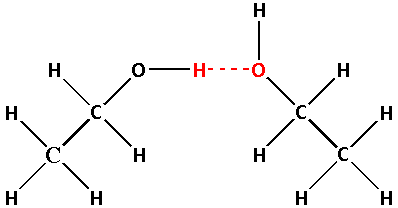
In dimethyl ether, the hydrogen atom is not bonded to the electronegative atom instead it is bonded to a carbon atom. Thus, hydrogen bonding is not possible in dimethyl ether.
Thus, ethyl alcohol is more soluble in water than dimethyl ether because alcohol contains hydrogen bonding.
Thus, the correct option is (B) alcohol contains hydrogen bonding.
Note: In ether, oxygen atoms are not forming hydrogen bonds. This makes ether soluble in water.
Hydrogen bonding is possible in ethyl alcohol. The bonding between an electronegative atom and a hydrogen atom bonded to another electronegative atom.
Complete step by step answer:
The structures of ethyl alcohol and dimethyl ether is as follows:
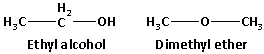
From the structures of ethyl alcohol and dimethyl ether, we can say that there is electronegativity difference in the bonded atoms. Thus, both ethyl alcohol and dimethyl ether are polar molecules. Water is also polar in nature. According to the rule ‘like dissolves like’, ethyl alcohol and dimethyl ether which are polar in nature are soluble in water which is also polar.
Now, we have to find out which amongst the two is more soluble in water. For this, we take into account the hydrogen bonding. The bonding between an electronegative atom and a hydrogen atom bonded to another electronegative atom like oxygen, nitrogen or fluorine is known as hydrogen bonding.
In ethyl alcohol, the hydrogen atom is bonded to an electronegative oxygen atom. Thus, hydrogen bonding is possible in ethyl alcohol. The hydrogen bonding in ethyl alcohol is shown in the following image.

In dimethyl ether, the hydrogen atom is not bonded to the electronegative atom instead it is bonded to a carbon atom. Thus, hydrogen bonding is not possible in dimethyl ether.
Thus, ethyl alcohol is more soluble in water than dimethyl ether because alcohol contains hydrogen bonding.
Thus, the correct option is (B) alcohol contains hydrogen bonding.
Note: In ether, oxygen atoms are not forming hydrogen bonds. This makes ether soluble in water.
Hydrogen bonding is possible in ethyl alcohol. The bonding between an electronegative atom and a hydrogen atom bonded to another electronegative atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




