
Ethyl ester $\xrightarrow[\text{excess}]{\text{C}{{\text{H}}_{\text{3}}}\text{MgBr}}$P. The product P will be
A.
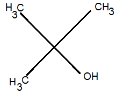
B.
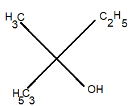
C.
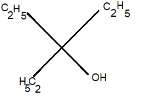
D.
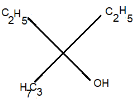




Answer
546k+ views
Hint:Esters as we know are the close relatives of aldehydes, ketones and carboxylic acids. They consist of a carbonyl group attached directly to an alkoxide (OR) group. Esters reacts with the organometallic compound (Grignard reagents) in the similar way as they react with aldehyde and ketones. But their mechanism is a little different.
Complete step-by-step answer:In the question, we are given with ethyl ester and this has to be reacted with Grignard reagent. We should also note that Grignard reagent is in excess, which means there are multiple moles of Grignard reagent available.
Let us discuss in brief what exactly the reaction is.
Firstly, 1 mole of Grignard reagent will react with ester to form a ketone. The ketone thus formed reacts with another mole of Grignard reagent to yield tertiary alcohol after protonation.
Let us now see the detail mechanism of the reaction:
Step 1: The given Grignard reagent $\left( C{{H}_{3}}MgBr \right)$will perform an addition reaction on ethyl ester $(C{{H}_{3}}COO{{C}_{2}}{{H}_{5}})$, forming C-C bond and breaking the old C-O pi bons. This produces an intermediate with a negatively charged oxygen. This step is similar with the addition of Grignard reagent to aldehydes and ketones.
Step 2: This step is the step of elimination reaction. The intermediate so formed, has a good leaving group $\left( -OC{{H}_{2}}C{{H}_{3}} \right)$. As a next step this leaving group leaves. In this complete step, we can say that there is reformation of C-O pi bond and breaking of C-O single bond. As a result, acetone is formed.
Step 3: The ketone (acetone) so formed reacts with another mole of Grignard reagent to bring out the addition reaction. In this step, the same process of step 1 is repeated. That is a C-C new bond is formed and C-O bond is broken. As a result. a tertiary alkoxide is produced.
Step 4: Finally, the protonation of this tertiary alkoxide forms tertiary alcohol
($1,1\text{dimethyl ethane-1-ol}$)
The diagrammatic representation of complete reaction is given below:
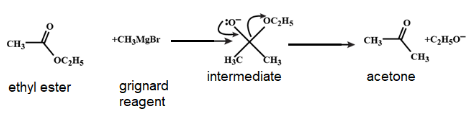
The acetone thus formed will react as follows:
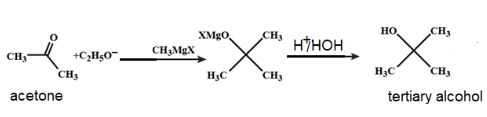
Therefore, the correct option is A.
Note: It should be noted that it is not practically feasible to isolate and obtain mere a ketone by the reaction of ester with only one mole of the available Grignard reagent. If we want to isolate ketone then this can be carried out with other organometallic reagents, for example organolithium reagent.
Complete step-by-step answer:In the question, we are given with ethyl ester and this has to be reacted with Grignard reagent. We should also note that Grignard reagent is in excess, which means there are multiple moles of Grignard reagent available.
Let us discuss in brief what exactly the reaction is.
Firstly, 1 mole of Grignard reagent will react with ester to form a ketone. The ketone thus formed reacts with another mole of Grignard reagent to yield tertiary alcohol after protonation.
Let us now see the detail mechanism of the reaction:
Step 1: The given Grignard reagent $\left( C{{H}_{3}}MgBr \right)$will perform an addition reaction on ethyl ester $(C{{H}_{3}}COO{{C}_{2}}{{H}_{5}})$, forming C-C bond and breaking the old C-O pi bons. This produces an intermediate with a negatively charged oxygen. This step is similar with the addition of Grignard reagent to aldehydes and ketones.
Step 2: This step is the step of elimination reaction. The intermediate so formed, has a good leaving group $\left( -OC{{H}_{2}}C{{H}_{3}} \right)$. As a next step this leaving group leaves. In this complete step, we can say that there is reformation of C-O pi bond and breaking of C-O single bond. As a result, acetone is formed.
Step 3: The ketone (acetone) so formed reacts with another mole of Grignard reagent to bring out the addition reaction. In this step, the same process of step 1 is repeated. That is a C-C new bond is formed and C-O bond is broken. As a result. a tertiary alkoxide is produced.
Step 4: Finally, the protonation of this tertiary alkoxide forms tertiary alcohol
($1,1\text{dimethyl ethane-1-ol}$)
The diagrammatic representation of complete reaction is given below:

The acetone thus formed will react as follows:

Therefore, the correct option is A.
Note: It should be noted that it is not practically feasible to isolate and obtain mere a ketone by the reaction of ester with only one mole of the available Grignard reagent. If we want to isolate ketone then this can be carried out with other organometallic reagents, for example organolithium reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




