
When ethyl ethanoate is treated with excess of MeMgBr followed by hydrolysis, the product is:


Answer
578.4k+ views
Hint: MeMgBr is a Grignard reagent and it reacts with many compounds due to its carbanionic property. It acts as a carbanion. To be able to solve this question, we need to find the structure of ethyl ethanoate first and then make the reactions.
Complete step by step solution:
- Ethyl ethanoate is an ester as the name suggests and its structure is of the type R-COOR. The structure of ethyl ethanoate can be shown as
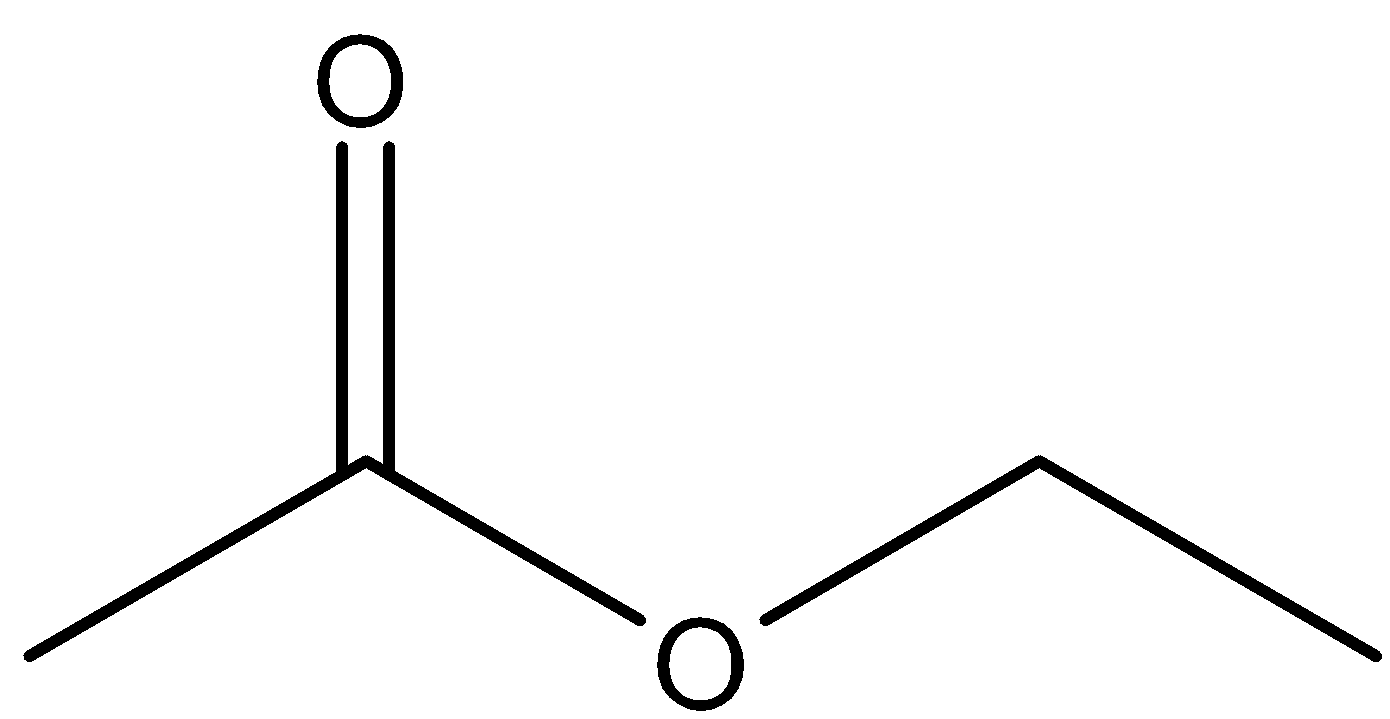
-It reacts with grignard’s reagent undergoing nucleophilic addition reaction attacking the –C=O bond and when the compound undergoes hydrolysis, the formation of alcohol takes place due to protonation. The reactions can be shown as

-First the rupture of –OR bond occurs as Grignard reagent breaks the bonds and itself gets associated with it and then the Grignard reagent reacts with the carbonyl group present in the compound. So 2 moles of Grignard reagent is consumed in the process.
-After that, hydrolysis occurs which is nothing but protonation and the hydrogen atom attaches itself to the oxygen atom to complete its octet and make the compound stable.
Therefore the final product obtained is shown and the correct option is A.
Note: When Grignard reagent attacks a compound, the reaction occurs such that the Grignard reagent is assumed to be a carbanion though it is not a carbanion. It reacts like a nucleophile and so the reaction is nucleophilic and not electrophilic.
Complete step by step solution:
- Ethyl ethanoate is an ester as the name suggests and its structure is of the type R-COOR. The structure of ethyl ethanoate can be shown as

-It reacts with grignard’s reagent undergoing nucleophilic addition reaction attacking the –C=O bond and when the compound undergoes hydrolysis, the formation of alcohol takes place due to protonation. The reactions can be shown as

-First the rupture of –OR bond occurs as Grignard reagent breaks the bonds and itself gets associated with it and then the Grignard reagent reacts with the carbonyl group present in the compound. So 2 moles of Grignard reagent is consumed in the process.
-After that, hydrolysis occurs which is nothing but protonation and the hydrogen atom attaches itself to the oxygen atom to complete its octet and make the compound stable.
Therefore the final product obtained is shown and the correct option is A.
Note: When Grignard reagent attacks a compound, the reaction occurs such that the Grignard reagent is assumed to be a carbanion though it is not a carbanion. It reacts like a nucleophile and so the reaction is nucleophilic and not electrophilic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




