
Ethylidene dichloride is obtained by the reaction of an excess of HCl with.
(A) Ethylene
(B) Acetylene
(C) Propane
(D) Methane
Answer
233.1k+ views
Hint: The structure of the Ethylidene dichloride is as follows.
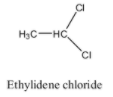
Ethylidene dichloride is also called Gem dihalides. Gem halogens mean the halogen atoms (chlorine) present on the same carbon atom in the molecule.
Complete step by step solution:
-Ethylidene chloride is also called 1,1-dichloroethane.
-We have to prepare Ethylidene dichloride by using an excess amount of HCl.
-Coming to the given options, option A, Ethylene. Alkenes generally form only monochloro derivatives with hydrochloric acid.
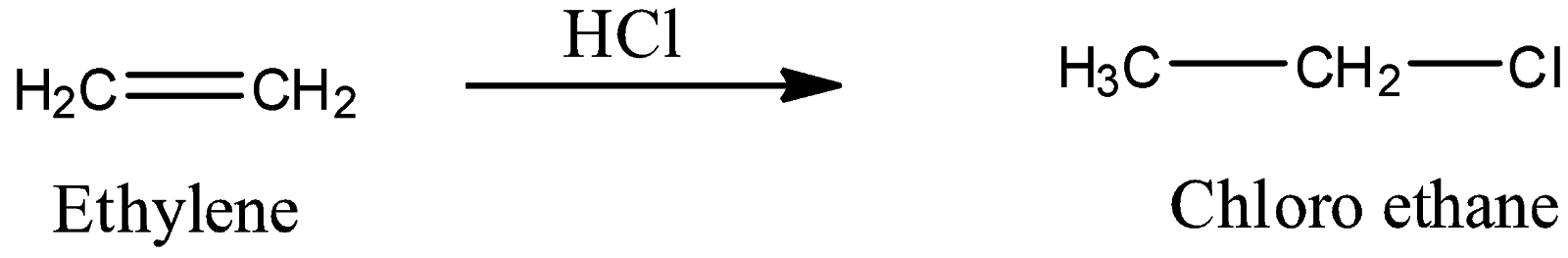
-So, option A is wrong.
-Coming to option B, Acetylene. Acetylene reacts with an excess amount of HCl and forms Ethylidene dichloride as the product. The reaction is as follows.
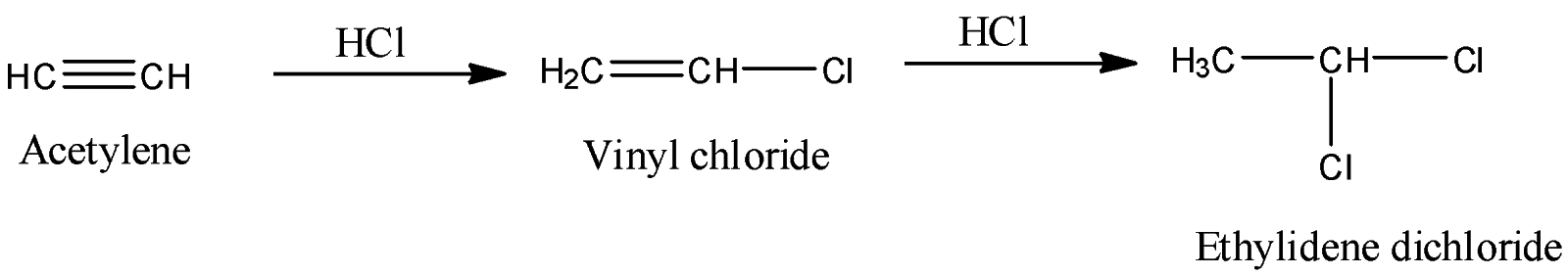
-It is a best example for electrophilic addition reaction.
-Coming to option C, Propane. Propane does not form Ethylidene dichloride with an excess amount of HCl. So, option C is wrong.
-Coming to option D, Methane. Methane does not form Ethylidene dichloride with an excess amount of HCl. So, option D is wrong.
-Therefore acetylene forms Ethylidene dichloride with an excess amount of HCl.
So, the correct option is (B).
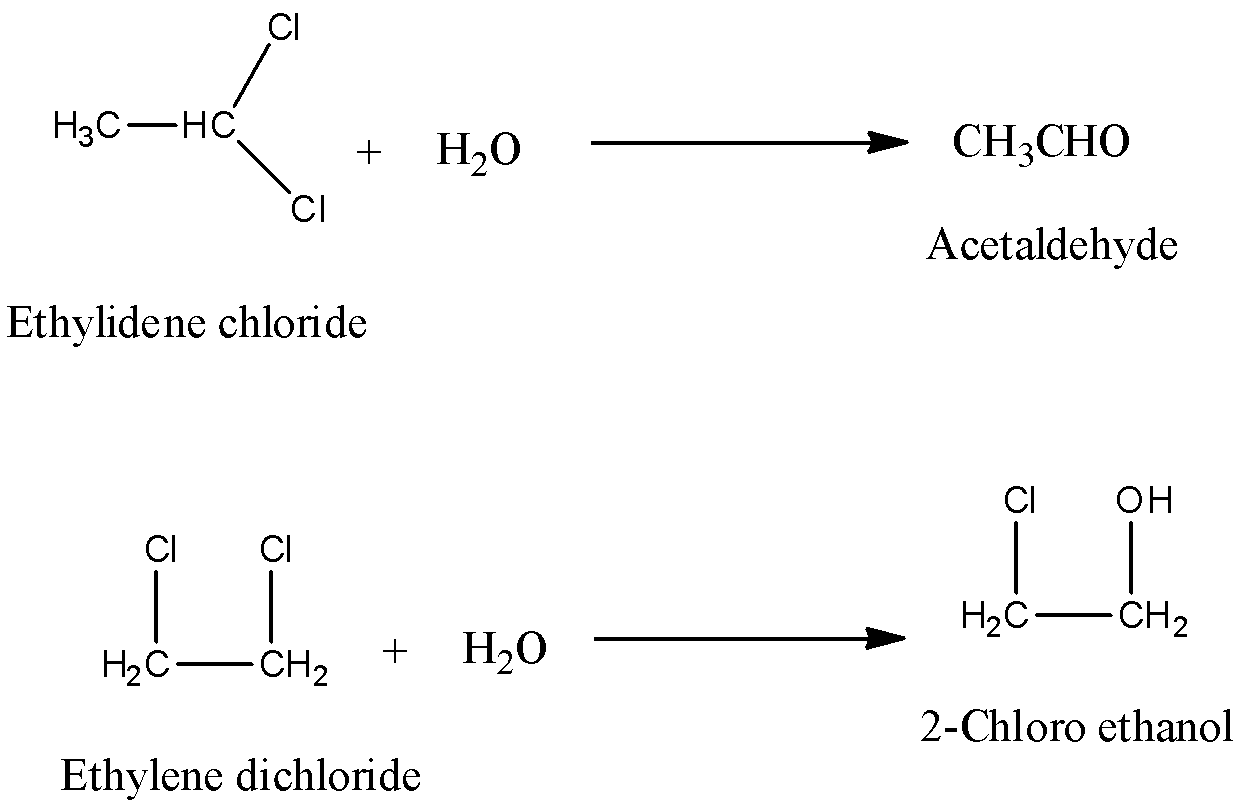
Note: Don’t be confused with Ethylidene chloride and ethylene dichloride. Both are not the same.
Ethylidene chloride and ethylene dichloride can be differentiated by reacting with water.
Ethylidene chloride forms acetaldehyde on reaction with water and ethylene dichloride forms 2-chloro ethanol on reaction with water.

Ethylidene dichloride is also called Gem dihalides. Gem halogens mean the halogen atoms (chlorine) present on the same carbon atom in the molecule.
Complete step by step solution:
-Ethylidene chloride is also called 1,1-dichloroethane.
-We have to prepare Ethylidene dichloride by using an excess amount of HCl.
-Coming to the given options, option A, Ethylene. Alkenes generally form only monochloro derivatives with hydrochloric acid.

-So, option A is wrong.
-Coming to option B, Acetylene. Acetylene reacts with an excess amount of HCl and forms Ethylidene dichloride as the product. The reaction is as follows.
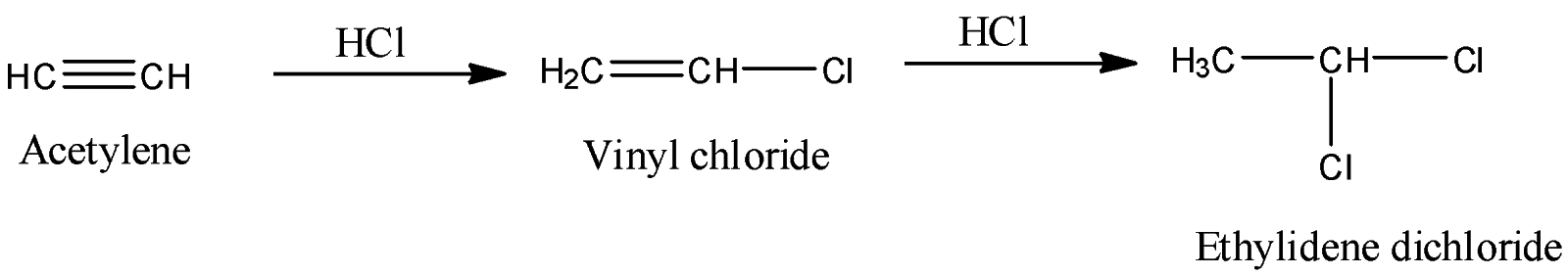
-It is a best example for electrophilic addition reaction.
-Coming to option C, Propane. Propane does not form Ethylidene dichloride with an excess amount of HCl. So, option C is wrong.
-Coming to option D, Methane. Methane does not form Ethylidene dichloride with an excess amount of HCl. So, option D is wrong.
-Therefore acetylene forms Ethylidene dichloride with an excess amount of HCl.
So, the correct option is (B).
Note: Don’t be confused with Ethylidene chloride and ethylene dichloride. Both are not the same.
Ethylidene chloride and ethylene dichloride can be differentiated by reacting with water.
Ethylidene chloride forms acetaldehyde on reaction with water and ethylene dichloride forms 2-chloro ethanol on reaction with water.

Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




