
Example of an aromatic ether is/ are:
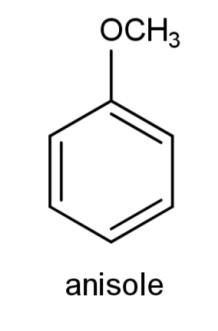
A.
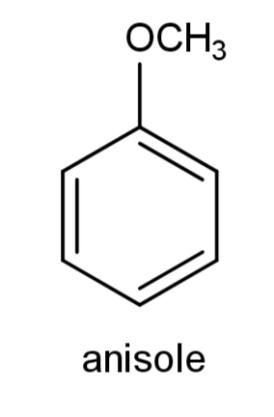
B.
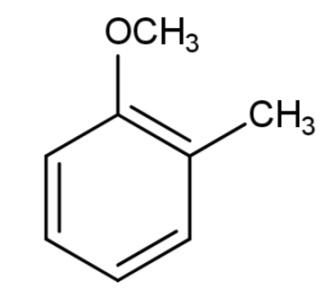
C.
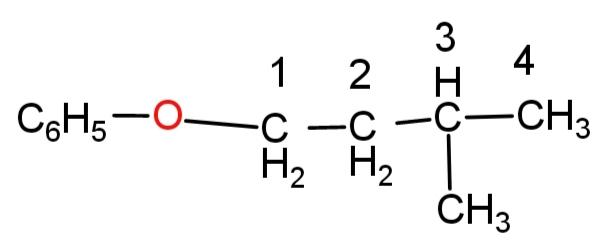
D. All of these



Answer
515.1k+ views
Hint: Ether is a functional group having R – O – R arrangement, where R is the alkyl or aryl groups that can be the same or different. Aromatic compounds are the compounds consisting of an alternated double and single bond arrangement that can lead to hyper conjugation, it also follows Huckel rule of aromaticity.
Complete answer:
We have been given various ethers from which we have to find the aromatic ethers. Ethers are a functional group in which oxygen is attached with two alkyl or aryl groups that can be the same or different. Aromaticity of any compound is the property to possess a ring that consists of complete delocalization of pi electrons. The ring should have an alternate conjugate system of single and double bonds to carry on the delocalization of electrons. The compound should be planar along with delocalization of pi electrons.
Among the given ethers, we have all of them containing a ring conjugated system of alternate single and double bonds as follows:
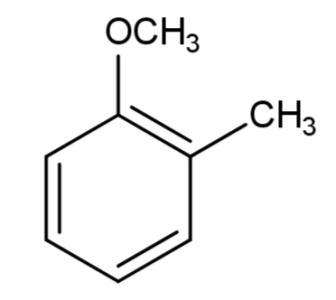
The compound having${{C}_{6}}{{H}_{5}}-O-C{{H}_{2}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ is also aromatic as it contains a 6 carbon ring with 5 hydrogen atoms making it a planar and aromatic molecule.
Hence, all the given ethers are aromatic. So, option D is correct.
Note:
Apart from being planar and having delocalization, the compound must follow $\left( 4n+2 \right)\pi $ electrons in the ring in order to be aromatic, where n is the integer = 0, 1, 2…etc. These conditions make the compound aromatic. Also the flame test can practically tell if it is aromatic or not. An aromatic compound burns with a smoky flame.
Complete answer:
We have been given various ethers from which we have to find the aromatic ethers. Ethers are a functional group in which oxygen is attached with two alkyl or aryl groups that can be the same or different. Aromaticity of any compound is the property to possess a ring that consists of complete delocalization of pi electrons. The ring should have an alternate conjugate system of single and double bonds to carry on the delocalization of electrons. The compound should be planar along with delocalization of pi electrons.
Among the given ethers, we have all of them containing a ring conjugated system of alternate single and double bonds as follows:


The compound having${{C}_{6}}{{H}_{5}}-O-C{{H}_{2}}-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ is also aromatic as it contains a 6 carbon ring with 5 hydrogen atoms making it a planar and aromatic molecule.
Hence, all the given ethers are aromatic. So, option D is correct.
Note:
Apart from being planar and having delocalization, the compound must follow $\left( 4n+2 \right)\pi $ electrons in the ring in order to be aromatic, where n is the integer = 0, 1, 2…etc. These conditions make the compound aromatic. Also the flame test can practically tell if it is aromatic or not. An aromatic compound burns with a smoky flame.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




