
What is an example of orbital hybridization?
Answer
490.2k+ views
Hint: Hybridization is the process of combining two atomic orbitals with the same energy levels to produce a degraded new kind of orbitals. Quantum mechanics is used to explain this intermixing. Only atomic orbitals with the same energy level may participate in hybridization, and both full and half-filled orbitals can participate in this process if their energies are equivalent.
Complete answer:
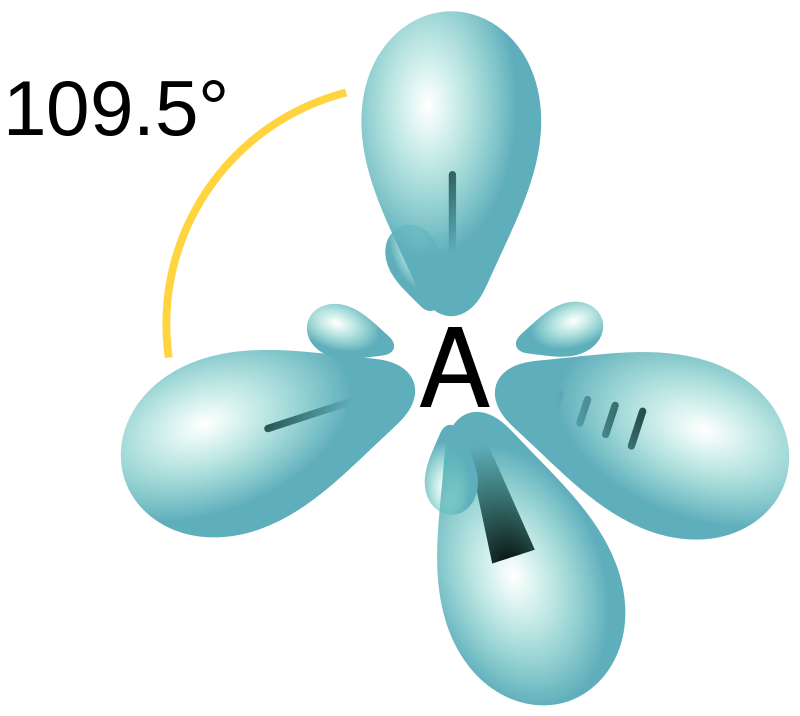
In valence bond theory, orbital hybridisation is the process of combining atomic orbitals to create new hybrid orbitals that are appropriate for electron pairing to form chemical bonds. For example, the valence-shell s orbital of a carbon atom that makes four single bonds mixes with three valence-shell p orbitals to produce four equivalent \[s{{p}^{3}}\] mixtures that are placed in a tetrahedral configuration around the carbon to connect to four distinct atoms. Hybrid orbitals are symmetrically arranged in space and are important in explaining molecular geometry and atomic bonding characteristics. Hybrid orbitals are usually created by combining atomic orbitals with similar energies.
Orbitals are a mathematical description of electron activity in molecules. This approach is based on atomic orbitals, which are comparable to those derived for the hydrogen atom, which is the only neutral atom for which the Schrödinger equation can be solved correctly. The atomic orbitals utilised in heavier elements, such as carbon, nitrogen, and oxygen, are the 2s and 2p orbitals, which are comparable to the excited state orbitals used in hydrogen.
The C hybrid orbital that generates each carbon–hydrogen bond in methane is characterised as \[s{{p}^{3}}\] hybridised because it contains 25% s and 75% p characters. This hybrid is described as an \[s{{p}^{3}}\] wavefunction of the type \[N\left( s\text{ }+\surd 3p\sigma \right)\] , where N is a normalisation constant (here $\dfrac{1}{2}$ ) and $p\sigma $ is a p orbital directed down the C-H axis to form a sigma bond, according to quantum mechanics. In this case, the coefficient ratio (often written as $\lambda $) is \[\surd 3\]. The ratio of p-character to s-character is \[{{\lambda }^{2}}=\text{ }3\] because the electron density associated with an orbital is proportional to the square of the wavefunction. \[{{N}^{2}}{{\lambda }^{2}}=\text{ }\dfrac{3}{4}\] is the p character or the weight of the p component.
Note:
Bonding orbitals derived from hybrid atomic orbitals can be thought of as localised molecular orbitals, which can be obtained by applying a mathematical transformation to the delocalized orbitals of molecular orbital theory. This translation of the orbitals keeps the overall many-electron wave function unaltered for molecules in the ground state. The delocalized orbital description for ground state total energy and electron density, as well as the molecular geometry that corresponds to the lowest total energy value, are therefore equal to the hybrid orbital description of the ground state.
Complete answer:
In valence bond theory, orbital hybridisation is the process of combining atomic orbitals to create new hybrid orbitals that are appropriate for electron pairing to form chemical bonds. For example, the valence-shell s orbital of a carbon atom that makes four single bonds mixes with three valence-shell p orbitals to produce four equivalent \[s{{p}^{3}}\] mixtures that are placed in a tetrahedral configuration around the carbon to connect to four distinct atoms. Hybrid orbitals are symmetrically arranged in space and are important in explaining molecular geometry and atomic bonding characteristics. Hybrid orbitals are usually created by combining atomic orbitals with similar energies.
Orbitals are a mathematical description of electron activity in molecules. This approach is based on atomic orbitals, which are comparable to those derived for the hydrogen atom, which is the only neutral atom for which the Schrödinger equation can be solved correctly. The atomic orbitals utilised in heavier elements, such as carbon, nitrogen, and oxygen, are the 2s and 2p orbitals, which are comparable to the excited state orbitals used in hydrogen.
The C hybrid orbital that generates each carbon–hydrogen bond in methane is characterised as \[s{{p}^{3}}\] hybridised because it contains 25% s and 75% p characters. This hybrid is described as an \[s{{p}^{3}}\] wavefunction of the type \[N\left( s\text{ }+\surd 3p\sigma \right)\] , where N is a normalisation constant (here $\dfrac{1}{2}$ ) and $p\sigma $ is a p orbital directed down the C-H axis to form a sigma bond, according to quantum mechanics. In this case, the coefficient ratio (often written as $\lambda $) is \[\surd 3\]. The ratio of p-character to s-character is \[{{\lambda }^{2}}=\text{ }3\] because the electron density associated with an orbital is proportional to the square of the wavefunction. \[{{N}^{2}}{{\lambda }^{2}}=\text{ }\dfrac{3}{4}\] is the p character or the weight of the p component.

Note:
Bonding orbitals derived from hybrid atomic orbitals can be thought of as localised molecular orbitals, which can be obtained by applying a mathematical transformation to the delocalized orbitals of molecular orbital theory. This translation of the orbitals keeps the overall many-electron wave function unaltered for molecules in the ground state. The delocalized orbital description for ground state total energy and electron density, as well as the molecular geometry that corresponds to the lowest total energy value, are therefore equal to the hybrid orbital description of the ground state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




