
Example(s) of super octet molecule is/are:
A. \[Cl{{F}_{3}}\]
B. \[PC{{l}_{5}}\]
C. \[I{{F}_{7}}\]
D. All of the above
Answer
596.7k+ views
Hint: As the name suggests, the octet rule is a chemical rule of thumb that reflects the observation that the main group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gas. A super octet molecule is also termed as an expanded octet molecule which occurs in special elements where their valence electrons accommodate more than eight electrons.
Complete-step- by- step answer:
Super octet or expanded octet can be seen usually in the third period or higher, especially with p-block elements and because they have d -orbitals that aren’t filled with electrons that can be used in bonding.
Now let us discuss all options step by step:
A. \[Cl{{F}_{3}}\] : As we know chlorine trifluoride is an interhalogen compound with the formula \[Cl{{F}_{3}}\]. Now as the number of electrons in the outermost shell of the central atom that is chlorine Cl are ten, (seven from its own and three from fluorine). Therefore, it has more than an octet of electrons.
We can see it in the structure:
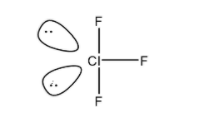
B. \[PC{{l}_{5}}\] : As we know, in the phosphorus pentachloride molecule the central atom phosphorus atom is bonded to five Cl atoms, hence having 10 bonding electrons and violating the octet rule means the d -orbitals participating in bonding with other atoms and hence a super octet molecule is produced.
Now, we will represent the molecule in the structure as:
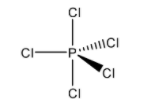
C. \[I{{F}_{7}}\] : Iodine heptafluoride has the molecular formula \[I{{F}_{7}}\]. Its outermost shell shares seven of its electrons with one electron from seven fluorine atoms. Therefore, the total number of electrons in the outermost shell of the central atom is 14 and hence a super octet molecule is produced.
So, now we will represent the molecule by its structure, i.e.
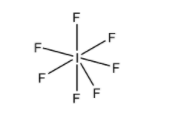
Now from the above, it is concluded that all given options are having more than eight electrons and hence the correct option is (D).
Note: Remember, do not confuse octet molecule with super octet molecule because octet molecule is the one which has eight electrons in its valence shell giving it the same electronic configuration as noble gas while super octet molecule has more than eight electrons in an ultimate shell around the central atom.
Complete-step- by- step answer:
Super octet or expanded octet can be seen usually in the third period or higher, especially with p-block elements and because they have d -orbitals that aren’t filled with electrons that can be used in bonding.
Now let us discuss all options step by step:
A. \[Cl{{F}_{3}}\] : As we know chlorine trifluoride is an interhalogen compound with the formula \[Cl{{F}_{3}}\]. Now as the number of electrons in the outermost shell of the central atom that is chlorine Cl are ten, (seven from its own and three from fluorine). Therefore, it has more than an octet of electrons.
We can see it in the structure:

B. \[PC{{l}_{5}}\] : As we know, in the phosphorus pentachloride molecule the central atom phosphorus atom is bonded to five Cl atoms, hence having 10 bonding electrons and violating the octet rule means the d -orbitals participating in bonding with other atoms and hence a super octet molecule is produced.
Now, we will represent the molecule in the structure as:

C. \[I{{F}_{7}}\] : Iodine heptafluoride has the molecular formula \[I{{F}_{7}}\]. Its outermost shell shares seven of its electrons with one electron from seven fluorine atoms. Therefore, the total number of electrons in the outermost shell of the central atom is 14 and hence a super octet molecule is produced.
So, now we will represent the molecule by its structure, i.e.

Now from the above, it is concluded that all given options are having more than eight electrons and hence the correct option is (D).
Note: Remember, do not confuse octet molecule with super octet molecule because octet molecule is the one which has eight electrons in its valence shell giving it the same electronic configuration as noble gas while super octet molecule has more than eight electrons in an ultimate shell around the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




