
Excess \[KCN\] is added to a solution of \[CuS{O_4}\] during the process of confirmation of \[Cu\] in the presence of \[Cd\] in group\[2\] . Pick out the correct statement with regards to the products formed.
This question has multiple correct options
A. \[{K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] is formed as the main product.
B. \[{\left( {CN} \right)_2}\]is one of the products obtained.
C. The main product is diamagnetic.
D. The hybridization of \[Cu\] in the product is \[s{p^3}\] .
Answer
571.8k+ views
Hint:Both the \[C{u^{2 + }}\]and \[C{d^{2 + }}\]cation form insoluble sulfides when treated with \[{H_2}S\] in acidic medium. The confirmatory test is achieved by treating the solution containing \[C{u^{2 + }}\] ion with \[KCN\] which forms a stable complex.
Complete step by step answer:The problem deals with the qualitative estimation of inorganic cations. After the removal of group \[1\] cations, the clear acidic solution is treated with \[{H_2}S\] gas or \[N{a_2}S\] solution. The group \[2\] cations are precipitated as sulfides under the given reaction conditions.
The precipitated sulfides of various cations have characteristic colors. The color of copper sulfide is black and the color of cadmium sulfide is yellow. The indication of the color gives a preliminary test for the presence of the cation.
The confirmatory test for the presence of \[C{u^{2 + }}\] ion is achieved by treating copper solution with excess \[KCN\]. The reaction of \[KCN\] and given solution of \[CuS{O_4}\] occurs in a stepwise manner. The sequence of reaction begins with the formation of cuprous cyanide and cyanogen gas.
The cuprous cyanide thus formed is unstable and dissolves in excess of \[KCN\]. The reaction is as follows:
\[CuS{O_4} + 2KCN \to Cu{(CN)_2} + {K_2}S{O_4}\]
$2Cu{(CN)_2} \to 2CuCN + CN - CN$
$CuCN + 3KCN \to {K_3}[Cu{(CN)_4}]$
Let us check the correctness of the given statements one by one.
A. \[{K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] is formed as the main product. This is incorrect statement as the main product is \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] and not \[{K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\]. The \[CuCN\] formed reacts with \[KCN\] to produce \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\].
B. \[{\left( {CN} \right)_2}\] is one of the products obtained. This is a correct statement as cuprous cyanide produced in the first step is unstable and undergoes decomposition to generate \[{\left( {CN} \right)_2}\] gas as one of the products of the reaction.
C. The main product is diamagnetic.
The main product is \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\]. The central metal ion is copper. Copper is an element in the periodic table with atomic number\[29\]. Its electronic configuration is\[\left[ {Ar} \right]3{d^{10}}4{s^1}\]. The oxidation state of copper in \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] is \[ + 1\]. The electron configuration is \[\left[ {Ar} \right]3{d^{10}}\]. The \[d\] orbitals are completely filled and all the electrons are paired. There is no unpaired electron. Thus the product is diamagnetic.
D. The hybridization of \[Cu\] in the product is\[s{p^3}\].
In the \[ + 1\] oxidation state of copper the inner \[d\] orbitals i.e. \[3d\]orbitals are filled up. The outer \[4s\] and \[4p\] orbitals are available for coordination with cyanide ligand. Thus the four cyanide ligands enter into the \[4s\] and \[4p\] orbitals. Thus the hybridization of \[Cu\] is \[s{p^3}\].
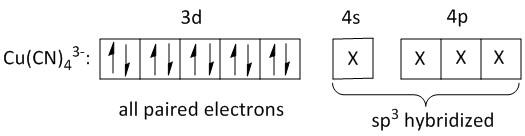
Hence statements B, C and D are correct.
Note:The similar complex formed with \[Cd\] is unstable, i.e.\[{K_3}\left[ {Cd{{\left( {CN} \right)}_4}} \right]\]. The complex product formed in this reaction is an anionic complex. The primary valency is satisfied by potassium ion and the secondary valency is satisfied by cyanide ion.
Complete step by step answer:The problem deals with the qualitative estimation of inorganic cations. After the removal of group \[1\] cations, the clear acidic solution is treated with \[{H_2}S\] gas or \[N{a_2}S\] solution. The group \[2\] cations are precipitated as sulfides under the given reaction conditions.
The precipitated sulfides of various cations have characteristic colors. The color of copper sulfide is black and the color of cadmium sulfide is yellow. The indication of the color gives a preliminary test for the presence of the cation.
The confirmatory test for the presence of \[C{u^{2 + }}\] ion is achieved by treating copper solution with excess \[KCN\]. The reaction of \[KCN\] and given solution of \[CuS{O_4}\] occurs in a stepwise manner. The sequence of reaction begins with the formation of cuprous cyanide and cyanogen gas.
The cuprous cyanide thus formed is unstable and dissolves in excess of \[KCN\]. The reaction is as follows:
\[CuS{O_4} + 2KCN \to Cu{(CN)_2} + {K_2}S{O_4}\]
$2Cu{(CN)_2} \to 2CuCN + CN - CN$
$CuCN + 3KCN \to {K_3}[Cu{(CN)_4}]$
Let us check the correctness of the given statements one by one.
A. \[{K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] is formed as the main product. This is incorrect statement as the main product is \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] and not \[{K_2}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\]. The \[CuCN\] formed reacts with \[KCN\] to produce \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\].
B. \[{\left( {CN} \right)_2}\] is one of the products obtained. This is a correct statement as cuprous cyanide produced in the first step is unstable and undergoes decomposition to generate \[{\left( {CN} \right)_2}\] gas as one of the products of the reaction.
C. The main product is diamagnetic.
The main product is \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\]. The central metal ion is copper. Copper is an element in the periodic table with atomic number\[29\]. Its electronic configuration is\[\left[ {Ar} \right]3{d^{10}}4{s^1}\]. The oxidation state of copper in \[{K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]\] is \[ + 1\]. The electron configuration is \[\left[ {Ar} \right]3{d^{10}}\]. The \[d\] orbitals are completely filled and all the electrons are paired. There is no unpaired electron. Thus the product is diamagnetic.
D. The hybridization of \[Cu\] in the product is\[s{p^3}\].
In the \[ + 1\] oxidation state of copper the inner \[d\] orbitals i.e. \[3d\]orbitals are filled up. The outer \[4s\] and \[4p\] orbitals are available for coordination with cyanide ligand. Thus the four cyanide ligands enter into the \[4s\] and \[4p\] orbitals. Thus the hybridization of \[Cu\] is \[s{p^3}\].

Hence statements B, C and D are correct.
Note:The similar complex formed with \[Cd\] is unstable, i.e.\[{K_3}\left[ {Cd{{\left( {CN} \right)}_4}} \right]\]. The complex product formed in this reaction is an anionic complex. The primary valency is satisfied by potassium ion and the secondary valency is satisfied by cyanide ion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




