
Explain, giving reasons for your answer, why Calcium is more reactive than Magnesium but less reactive than Sodium?
Answer
493.5k+ views
Hint: In order to solve this question, we should know about reactivity series. The reactivity series is the arrangement of elements according to their reactivity. It is also known as the activity series .It also tells about the element which oxidizes more rapidly to form positive ions.
Complete Step By Step Answer:
The reactivity series is the series in which elements are arranged according to their reactivity, how fast they react. The more reactive elements are placed on the top while least on the bottom. More reactive element loses electrons greatly and forms positive ions. Whereas the least reactive element loses electrons slowly as compared to the highly reactive element. The reactivity series of the element is:
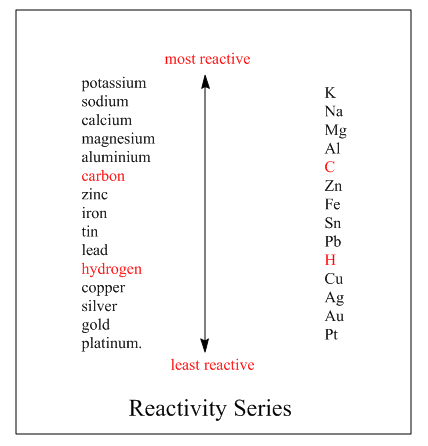
On moving up the series the reactivity of the elements increases, thus the element becomes more reactive. Here Potassium is the most reactive element than other as it is on the top of the series whereas Platinum on the bottom, thus least reactive element than others. As from series we can see calcium is above the magnesium in the reactivity series. Hence it is more reactive than magnesium. On the other hand, it is below the sodium in the reactivity series, so it is less reactive than sodium.
Note:
The reactivity series also tells about the elements which corrode or tarnish more rapidly, also require more energy to be isolated from their compounds and which element can become stronger reducing agents.
Complete Step By Step Answer:
The reactivity series is the series in which elements are arranged according to their reactivity, how fast they react. The more reactive elements are placed on the top while least on the bottom. More reactive element loses electrons greatly and forms positive ions. Whereas the least reactive element loses electrons slowly as compared to the highly reactive element. The reactivity series of the element is:

On moving up the series the reactivity of the elements increases, thus the element becomes more reactive. Here Potassium is the most reactive element than other as it is on the top of the series whereas Platinum on the bottom, thus least reactive element than others. As from series we can see calcium is above the magnesium in the reactivity series. Hence it is more reactive than magnesium. On the other hand, it is below the sodium in the reactivity series, so it is less reactive than sodium.
Note:
The reactivity series also tells about the elements which corrode or tarnish more rapidly, also require more energy to be isolated from their compounds and which element can become stronger reducing agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




