
Explain impurity defect in stainless steel with diagram.
Answer
595.2k+ views
Hint: Start with the components of stainless steel. Compare the sizes of atoms of different elements. The smallest one causes impurity.
Complete step by step solution:
Let us understand what it means by impurity defects in a crystalline structure.
Defects in a crystalline structure are completely natural and we humans have been successful in removing them to a certain extent, but not all. Defects are actually those which disturb both the long range and short range order in a crystalline solid. They are of the following types:
- Point defects
These are the defects in or around a specific atom, molecule or ion.
- Line defects
This is a defect in the entire row or column of a crystalline structure.
Impurity defect is one of the many point defects. As its name suggests, in this type of defect the crystalline solid structure of a particular entity contains trace or minimal amounts of another entity. The latter therefore becomes impure in the former.
We explain this with the help of stainless steel.
Well, stainless steel is composed of iron and at least $(10.5-11)%$ of chromium. It can be more according to the specified requirements, but this is the minimal concentration. It also contains other elements in trace amounts like carbon, nickel, molybdenum, titanium etc.
The sizes of all the metal atoms mentioned above are to some extent similar to each other if not same. In a crystal lattice these atoms can form rigid structures due to this similarity. But one element is clearly the “odd one out”, and that is carbon.
Carbon belongs to the second period and fourteenth group of the periodic table. It is therefore a metalloid by nature. It is also the smallest in its period as it is the topmost element and carbon even lacks a d-orbital. The size of this atom perfectly fits the voids created by the iron element. The carbon atom thus remains in interstitial areas. It is called an impurity because its distribution is not regular and therefore it disturbs both the short and long range order of the whole crystalline structure. A pictorial representation supporting these theories is pasted below:
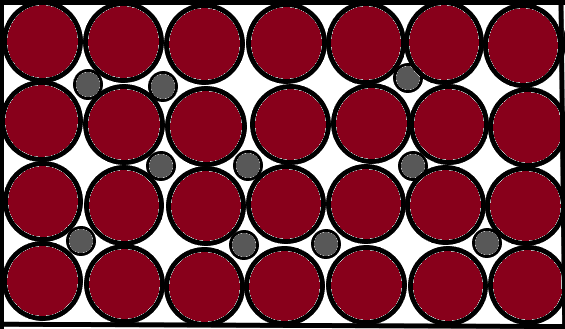
(The atoms in red are those of iron and the grey ones are carbon atoms which are the impurities.)
Note: You cannot consider the atoms of other elements in a crystal lattice as impurity as long as they do not disturb the order of arrangements of the whole structure. As in this case, the elements such as chromium and titanium cannot be taken as impurities because they maintain the structure of the crystal lattice.
Impurities are not as bad as their name suggests. In fact many special properties can be introduced into solids through impurities that make them more useful to us.
Complete step by step solution:
Let us understand what it means by impurity defects in a crystalline structure.
Defects in a crystalline structure are completely natural and we humans have been successful in removing them to a certain extent, but not all. Defects are actually those which disturb both the long range and short range order in a crystalline solid. They are of the following types:
- Point defects
These are the defects in or around a specific atom, molecule or ion.
- Line defects
This is a defect in the entire row or column of a crystalline structure.
Impurity defect is one of the many point defects. As its name suggests, in this type of defect the crystalline solid structure of a particular entity contains trace or minimal amounts of another entity. The latter therefore becomes impure in the former.
We explain this with the help of stainless steel.
Well, stainless steel is composed of iron and at least $(10.5-11)%$ of chromium. It can be more according to the specified requirements, but this is the minimal concentration. It also contains other elements in trace amounts like carbon, nickel, molybdenum, titanium etc.
The sizes of all the metal atoms mentioned above are to some extent similar to each other if not same. In a crystal lattice these atoms can form rigid structures due to this similarity. But one element is clearly the “odd one out”, and that is carbon.
Carbon belongs to the second period and fourteenth group of the periodic table. It is therefore a metalloid by nature. It is also the smallest in its period as it is the topmost element and carbon even lacks a d-orbital. The size of this atom perfectly fits the voids created by the iron element. The carbon atom thus remains in interstitial areas. It is called an impurity because its distribution is not regular and therefore it disturbs both the short and long range order of the whole crystalline structure. A pictorial representation supporting these theories is pasted below:

(The atoms in red are those of iron and the grey ones are carbon atoms which are the impurities.)
Note: You cannot consider the atoms of other elements in a crystal lattice as impurity as long as they do not disturb the order of arrangements of the whole structure. As in this case, the elements such as chromium and titanium cannot be taken as impurities because they maintain the structure of the crystal lattice.
Impurities are not as bad as their name suggests. In fact many special properties can be introduced into solids through impurities that make them more useful to us.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




