
Explain Moeller diagram.
Answer
477k+ views
Hint: The Moeller diagram is a chemical tool for calculating an atom's electronic configuration based on its atomic number (Z). It's a visual and mnemonic way for learning Madelung's rule, or how to write an element's electron configuration. It is defined by drawing diagonals between the columns of the orbitals and determining the proper order of the same for an atom by following the direction of the arrow. The Moeller diagram governs the electrical arrangement of the components. It is founded on the idea of Aufbau.
Complete answer:
The Aufbau principle governs how electrons are filled in an atom's atomic orbitals while it is in its ground state. According to this theory, electrons are filled into atomic orbitals in sequence of increasing orbital energy level. According to the Aufbau principle, the lowest energy atomic orbitals are occupied first, followed by the higher energy levels. The Moeller diagram is shown below.
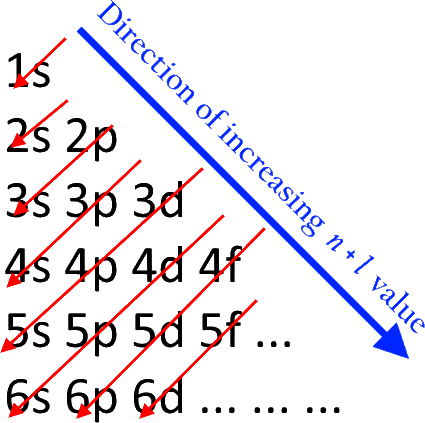
The word 'Aufbau' has German roots and essentially translates to 'build up' or 'construct.' The sequence in which atomic orbitals are filled is depicted in the diagram below. The main quantum number is ‘n,' while the azimuthal quantum number is ‘l.'
The Aufbau principle may be used to figure out where electrons are in an atom and what energy levels they correspond to. Carbon, for example, has six electrons and has an electrical structure . It's crucial to remember that each orbital can only carry two electrons (as per the Pauli exclusion principle). In addition, the way electrons are filled into orbitals in a single subshell must adhere to Hund's rule, which states that every orbital in a given subshell must be single-occupied by electrons before any two electrons couple up in an orbital.
The Aufbau principle states that electrons will inhabit the lowest-energy orbitals first. This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled. The (n+l) rule may be used to identify the sequence in which the energy of orbitals rises, with the sum of the primary and azimuthal quantum numbers determining the orbital energy level.
Lower orbital energies correlate to lower (n+l) values. When two orbitals have the same (n+l) values, the orbital with the lower n value is said to have less energy.
Note:
Other atomic physics concepts, such as Hund's rule and the Pauli exclusion principle, help to explain electron behaviour. If numerous orbitals of the same energy are accessible, electrons will occupy various orbitals singly before any are occupied twice, according to Hund's rule. If double occupancy occurs, the Pauli exclusion principle dictates that electrons in the same orbital have distinct spins ($\dfrac{+1}{2}$ and $\dfrac{-1}{2}$).
Complete answer:
The Aufbau principle governs how electrons are filled in an atom's atomic orbitals while it is in its ground state. According to this theory, electrons are filled into atomic orbitals in sequence of increasing orbital energy level. According to the Aufbau principle, the lowest energy atomic orbitals are occupied first, followed by the higher energy levels. The Moeller diagram is shown below.

The word 'Aufbau' has German roots and essentially translates to 'build up' or 'construct.' The sequence in which atomic orbitals are filled is depicted in the diagram below. The main quantum number is ‘n,' while the azimuthal quantum number is ‘l.'
The Aufbau principle may be used to figure out where electrons are in an atom and what energy levels they correspond to. Carbon, for example, has six electrons and has an electrical structure . It's crucial to remember that each orbital can only carry two electrons (as per the Pauli exclusion principle). In addition, the way electrons are filled into orbitals in a single subshell must adhere to Hund's rule, which states that every orbital in a given subshell must be single-occupied by electrons before any two electrons couple up in an orbital.
The Aufbau principle states that electrons will inhabit the lowest-energy orbitals first. This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled. The (n+l) rule may be used to identify the sequence in which the energy of orbitals rises, with the sum of the primary and azimuthal quantum numbers determining the orbital energy level.
Lower orbital energies correlate to lower (n+l) values. When two orbitals have the same (n+l) values, the orbital with the lower n value is said to have less energy.
Note:
Other atomic physics concepts, such as Hund's rule and the Pauli exclusion principle, help to explain electron behaviour. If numerous orbitals of the same energy are accessible, electrons will occupy various orbitals singly before any are occupied twice, according to Hund's rule. If double occupancy occurs, the Pauli exclusion principle dictates that electrons in the same orbital have distinct spins ($\dfrac{+1}{2}$ and $\dfrac{-1}{2}$).
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Trending doubts
What is meant by exothermic and endothermic reactions class 11 chemistry CBSE

Which animal has three hearts class 11 biology CBSE

10 examples of friction in our daily life

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

1 Quintal is equal to a 110 kg b 10 kg c 100kg d 1000 class 11 physics CBSE

Difference Between Prokaryotic Cells and Eukaryotic Cells




