
Explain Siemens-Martin’s open hearth process for manufacture of steel with diagrams.
Answer
590.7k+ views
Hint: This is a process of metallurgy during manufacturing of steel. The Metallurgy and isolation should be such that it is commercially feasible and chemically feasible. Siemens-Martin process is a steel making technique that for the major part of all steel made in the world. The process developed from the old processes which are using the waste heat given off the furnace, directing the fumes from the furnace through a brick work.
Complete step by step answer:
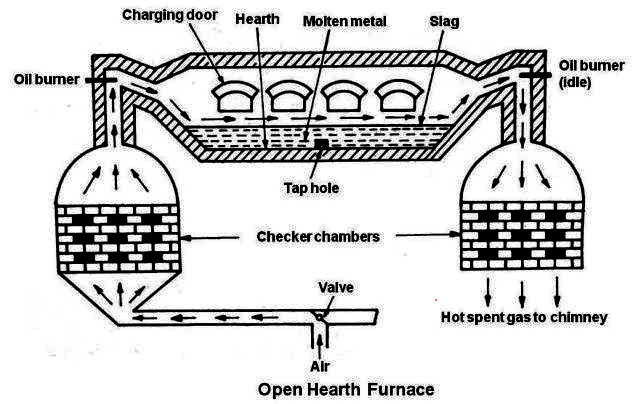
Open hearth process is also known as Siemens-Martin process.
The metallurgy process of iron by using the principle of open hearth process as follows,
The impurities present in cast iron are removed by hematite.
The percentage of carbon is decreased by adding scrap iron.
The heat required for the process is obtained by burning heated producer gas by regeneration of heat economy depending upon the impurities the lining of hearth is acidic or basic lining.
The percentage of carbon maintained by adding the required amount of spiegeleisen, which is an alloy of iron and used in the manufacture of steel.
The reactions during the manufacture of steel are,
$\begin{align}
& 3C+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3C{{O}_{2}} \\
& 3S+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3S{{O}_{2}} \\
& 3Si+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3Si{{O}_{2}} \\
& Si{{O}_{2}}+CaO\to CaSi{{O}_{3}} \\
\end{align}$
If phosphorus is present,
\[\begin{align}
& 6P+5F{{e}_{2}}{{O}_{3}}\to 10Fe+3{{P}_{2}}{{O}_{5}} \\
& {{P}_{2}}{{O}_{5}}+3CaO\to C{{a}_{3}}{{(P{{O}_{4}})}_{2}} \\
\end{align}\]
The advantages of open hearth process are as follows:
The quality of steel is uniform.
This process is easily controllable.
The loss of iron from iron ore is less, because of there is no blast of air is passed
The batch size in the open hearth process is very large compared to other processes.
Note:Though the open-hearth process has been almost completely replaced in most industrialized countries by the basic oxygen process and the electric arc furnace, it nevertheless accounts for about one-sixth of all steel produced worldwide.
Complete step by step answer:
Open hearth process is also known as Siemens-Martin process.
The metallurgy process of iron by using the principle of open hearth process as follows,
The impurities present in cast iron are removed by hematite.
The percentage of carbon is decreased by adding scrap iron.
The heat required for the process is obtained by burning heated producer gas by regeneration of heat economy depending upon the impurities the lining of hearth is acidic or basic lining.
The percentage of carbon maintained by adding the required amount of spiegeleisen, which is an alloy of iron and used in the manufacture of steel.
The reactions during the manufacture of steel are,
$\begin{align}
& 3C+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3C{{O}_{2}} \\
& 3S+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3S{{O}_{2}} \\
& 3Si+2F{{e}_{2}}{{O}_{3}}\to 4Fe+3Si{{O}_{2}} \\
& Si{{O}_{2}}+CaO\to CaSi{{O}_{3}} \\
\end{align}$
If phosphorus is present,
\[\begin{align}
& 6P+5F{{e}_{2}}{{O}_{3}}\to 10Fe+3{{P}_{2}}{{O}_{5}} \\
& {{P}_{2}}{{O}_{5}}+3CaO\to C{{a}_{3}}{{(P{{O}_{4}})}_{2}} \\
\end{align}\]

The advantages of open hearth process are as follows:
The quality of steel is uniform.
This process is easily controllable.
The loss of iron from iron ore is less, because of there is no blast of air is passed
The batch size in the open hearth process is very large compared to other processes.
Note:Though the open-hearth process has been almost completely replaced in most industrialized countries by the basic oxygen process and the electric arc furnace, it nevertheless accounts for about one-sixth of all steel produced worldwide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




