
Explain the adsorption theory of catalysis.
Answer
587.7k+ views
Hint: The heterogeneous catalysis is considered as the surface phenomena in which the catalytic activity of the catalyst is localized on the surface of the catalyst. The catalyst provides the surface and the reaction is explained based on adsorption theory. The reactant diffuses on the surface of the catalyst and adsorbs on the active sites of the catalyst. The adsorption results in the formation of an intermediate which breaks down into the product. The product forms desorbs from the surface of the catalyst and makes it available for more reaction.
Complete answer:
-When the catalyst is in a different phase than the reactant, it is called the heterogeneous catalyst. Such reactions are called heterogeneous catalytic reactions.
-In heterogeneous catalysis, the catalyst is generally solid and the reactants are generally in the gaseous phase.
-The mechanism for the heterogeneous catalysis can be explained based on adsorption theory. According to the adsorption theory, the surface of the catalyst has the free valencies which act as the site for the chemical force attraction. The reactants are in a gaseous state or the solution is adsorbed on the surface of the solid catalyst. As the reactant molecules are adsorbed, the bonds get weakened and the reaction can proceed quickly because the bonds are more quickly broken.
-Heterogeneous catalysis is a surface phenomenon. It involves the following steps:
1) Diffusion of the reactant to the surface of the catalyst.
2) Adsorption of the molecules of the reactant at the active sites.
3) The occurrence of the chemical reactions on the surface of the catalyst through the formation of an intermediate.
4) Desorption of product molecules from the surface and making a surface available again for the more reactions to takes place.
5) Diffusion of product away from the surface of the catalyst.
The mechanism for the adsorption of heterogeneous catalysis is as shown below:
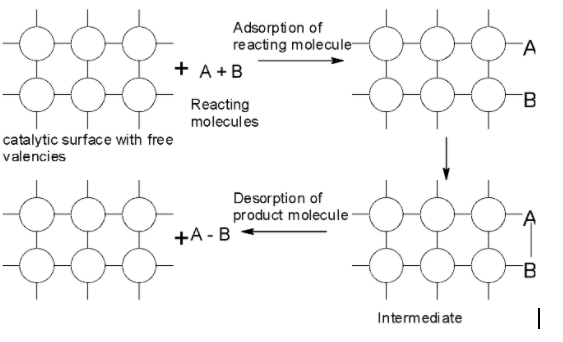
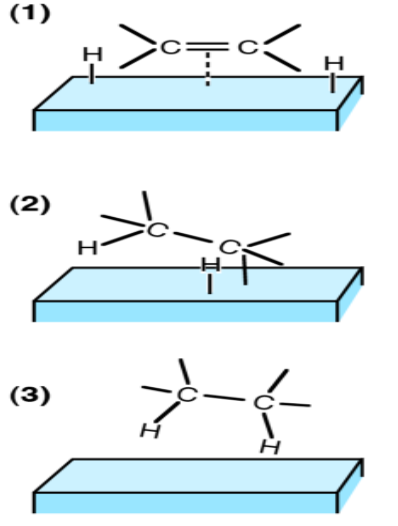
One of the most important examples of heterogeneous catalysis is the addition of ${{\text{H}}_{\text{2}}}$ the carbon-carbon double bond $\text{(C=C)}$ of organic compounds to form a $\text{C-C}$ single bond. This is called the catalytic hydrogenation reaction. The simplest hydrogenation reaction can be depicted as:
$\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}_{\text{(g)}}\text{+}{{\text{H}}_{\text{2}}}_{\text{(g)}}\to \text{C}{{\text{H}}_{\text{3}}}\text{-C}{{\text{H}}_{\text{3}}}$
In the absence of a catalyst, the reaction occurs very slowly. However, at high pressure in presence of hydrogen and the presence of finely divided nickel, palladium, or platinum as a catalyst, the reaction becomes rapid even at the ordinary temperature. The catalyzed reaction is believed to follow the adsorption theory. The following is considered as the pathways for the reaction:
Note:
Adsorption is an exothermic process, thus the heat generated during the adsorption is further used to break or weaken the bonds between the reactants, which favours the formation of new bonds. The homogeneous catalysis is explained based on intermediate compound formation. Here reactant and catalyst are in the same phase.
Complete answer:
-When the catalyst is in a different phase than the reactant, it is called the heterogeneous catalyst. Such reactions are called heterogeneous catalytic reactions.
-In heterogeneous catalysis, the catalyst is generally solid and the reactants are generally in the gaseous phase.
-The mechanism for the heterogeneous catalysis can be explained based on adsorption theory. According to the adsorption theory, the surface of the catalyst has the free valencies which act as the site for the chemical force attraction. The reactants are in a gaseous state or the solution is adsorbed on the surface of the solid catalyst. As the reactant molecules are adsorbed, the bonds get weakened and the reaction can proceed quickly because the bonds are more quickly broken.
-Heterogeneous catalysis is a surface phenomenon. It involves the following steps:
1) Diffusion of the reactant to the surface of the catalyst.
2) Adsorption of the molecules of the reactant at the active sites.
3) The occurrence of the chemical reactions on the surface of the catalyst through the formation of an intermediate.
4) Desorption of product molecules from the surface and making a surface available again for the more reactions to takes place.
5) Diffusion of product away from the surface of the catalyst.
The mechanism for the adsorption of heterogeneous catalysis is as shown below:

One of the most important examples of heterogeneous catalysis is the addition of ${{\text{H}}_{\text{2}}}$ the carbon-carbon double bond $\text{(C=C)}$ of organic compounds to form a $\text{C-C}$ single bond. This is called the catalytic hydrogenation reaction. The simplest hydrogenation reaction can be depicted as:
$\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}_{\text{(g)}}\text{+}{{\text{H}}_{\text{2}}}_{\text{(g)}}\to \text{C}{{\text{H}}_{\text{3}}}\text{-C}{{\text{H}}_{\text{3}}}$
In the absence of a catalyst, the reaction occurs very slowly. However, at high pressure in presence of hydrogen and the presence of finely divided nickel, palladium, or platinum as a catalyst, the reaction becomes rapid even at the ordinary temperature. The catalyzed reaction is believed to follow the adsorption theory. The following is considered as the pathways for the reaction:

Note:
Adsorption is an exothermic process, thus the heat generated during the adsorption is further used to break or weaken the bonds between the reactants, which favours the formation of new bonds. The homogeneous catalysis is explained based on intermediate compound formation. Here reactant and catalyst are in the same phase.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




