
Explain the anomalous expansion of water and draw the graph of the density of water v/s the temperature.
Answer
602.1k+ views
Hint: The anomalous properties of water is that the behaviour of water is different when compared to other liquids. When water is heated from ${{0}^{\circ }}C$ it contracts instead of expanding till it reaches a temperature of ${{4}^{\circ }}C$ as a result the density of water will be less in this temperature range. So, the ice will float above the water surface.
Complete step-by-step answer:
Ice is water in its solid phase, it has the same composition of hydrogen and oxygen atoms present in water. Usually, the density of water increases as temperature decreases. But water shows an anomalous behaviour at ${{4}^{\circ }}C$, if we decrease the temperature below ${{4}^{\circ }}C$ the density keeps on decreasing instead of increasing. This is why ice at ${{0}^{\circ }}C$ is less dense than water.
What differs in ice is that the structure of water molecules in liquid form and water molecules in solid form differs. There is a regular pattern in the arrangement of water molecules in the solid phase (ice) while there is no definite pattern of molecules in the liquid phase. As the solid phase molecules attain a regular structure, the molecules spread out, which in turn decreases the density of the ice.
A graph depicting the anomalous behaviour of water is given below, notice how the density drops from ${{4}^{\circ }}C$ to ${{0}^{\circ }}C$.
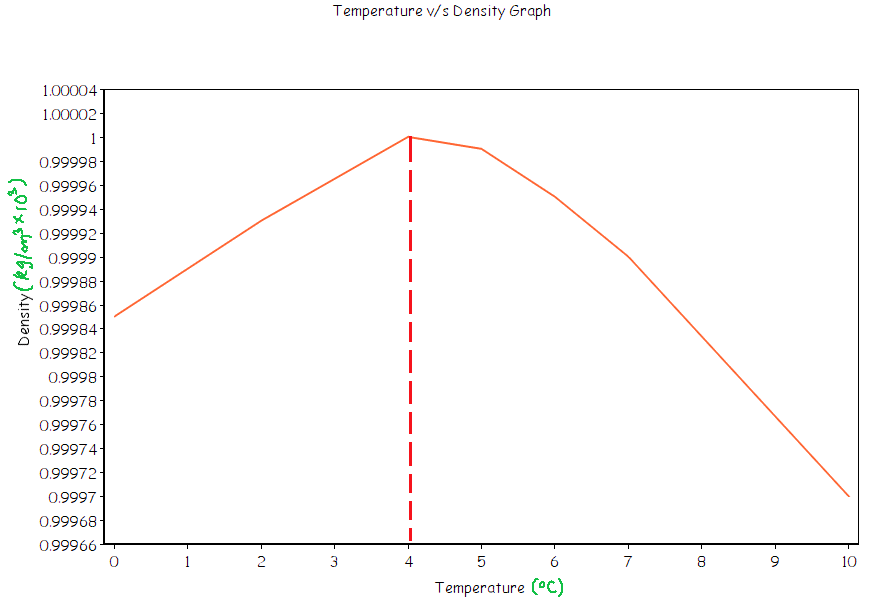
Note: This anomalous behaviour of water is responsible for life on earth because the aquatic plants and animals in water bodies are saved from freezing due to the freezing of only the top layer of the water bodies.
This property also has some disadvantages. In colder countries where the pipelines which transport water get frozen and due to the anomalous property of water, the pipes sometimes burst due to excess pressure.
The instrument to measure the density of a liquid is called a hydrometer. In liquid water, each molecule of hydrogen is bonded to approximately 3.4 other water molecules. In ice, each molecule of hydrogen is bonded to 4 other water molecules.
Complete step-by-step answer:
Ice is water in its solid phase, it has the same composition of hydrogen and oxygen atoms present in water. Usually, the density of water increases as temperature decreases. But water shows an anomalous behaviour at ${{4}^{\circ }}C$, if we decrease the temperature below ${{4}^{\circ }}C$ the density keeps on decreasing instead of increasing. This is why ice at ${{0}^{\circ }}C$ is less dense than water.
What differs in ice is that the structure of water molecules in liquid form and water molecules in solid form differs. There is a regular pattern in the arrangement of water molecules in the solid phase (ice) while there is no definite pattern of molecules in the liquid phase. As the solid phase molecules attain a regular structure, the molecules spread out, which in turn decreases the density of the ice.
A graph depicting the anomalous behaviour of water is given below, notice how the density drops from ${{4}^{\circ }}C$ to ${{0}^{\circ }}C$.

Note: This anomalous behaviour of water is responsible for life on earth because the aquatic plants and animals in water bodies are saved from freezing due to the freezing of only the top layer of the water bodies.
This property also has some disadvantages. In colder countries where the pipelines which transport water get frozen and due to the anomalous property of water, the pipes sometimes burst due to excess pressure.
The instrument to measure the density of a liquid is called a hydrometer. In liquid water, each molecule of hydrogen is bonded to approximately 3.4 other water molecules. In ice, each molecule of hydrogen is bonded to 4 other water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




