
Explain the following reactions:
(i) Diazotization
(ii) Carbylamine reaction
Answer
565.5k+ views
Hint:. The answer for this question is based on the concept of reactions of organic chemistry where the diazotization involves treatment of primary aromatic amines with nitrous acid and carbylamine reaction involves reaction of primary amine with chloroform and base.
Complete step by step answer:
The concepts of several named reactions and also some of the basic reactions like addition reactions, substitution reactions, elimination reactions, halogenations, cyanation etc are familiar to us from the chapters of organic chemistry.
We shall now see what is diazotization and carbylamine reaction in detailed form.
(i) Diazotization is the reaction in which aromatic amines that are the primary amines when treated with nitrous acid and mineral acid produce diazonium salt with the release of water as by product. The reaction in detail is as shown below:
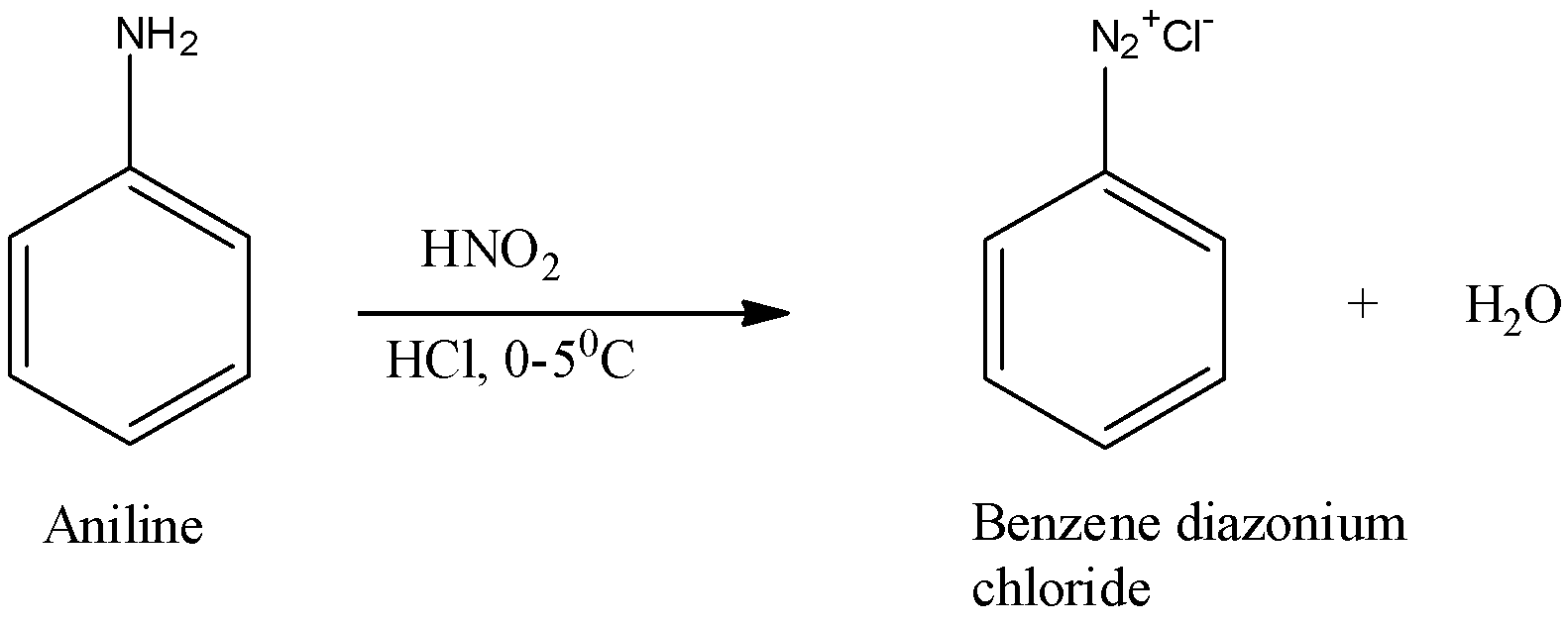
Here, aniline is the primary amine and when this reacts with nitrous acid, it forms benzene diazonium chloride along with water as by product.
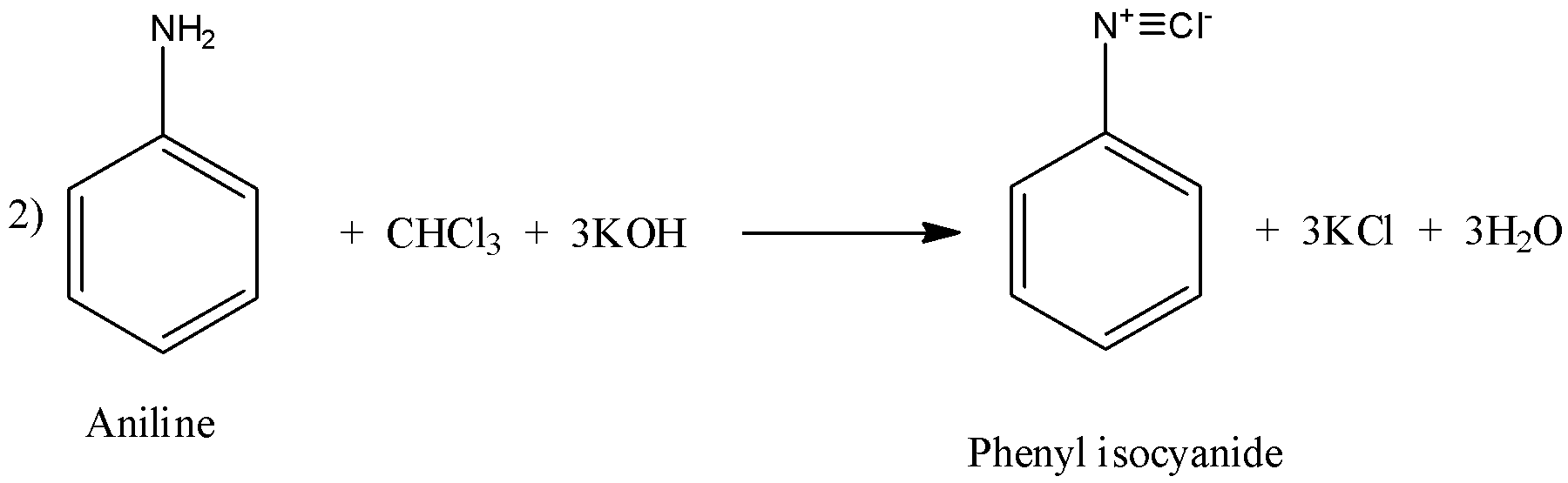
(ii) Carbylamine reaction is the one where primary amine reacts with chloroform and base to give isocyanide compounds.
The example for this reaction can be of aliphatic amines or aromatic amines as shown below:
\[\text{1) C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}\text{+CHC}{{\text{l}}_{3}}\text{+3KOH}\to \text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{NC+3KCl+3}{{\text{H}}_{2}}\text{O}\]
In the above reaction 1), the aliphatic primary amine that is ethyl amine reacts with chloroform and base to give ethyl isocyanide and by product of salt and water.
Similarly, in reaction 2), primary aromatic amine that is aniline reacts with chloroform and base to give phenyl isocyanide and similar products.
Note: Note that diazotization reactions are to be carried out at lower temperatures because if we employ higher temperature then the salts form others by products and thus result in the formation of phenol by the reaction with water at higher temperature.
Complete step by step answer:
The concepts of several named reactions and also some of the basic reactions like addition reactions, substitution reactions, elimination reactions, halogenations, cyanation etc are familiar to us from the chapters of organic chemistry.
We shall now see what is diazotization and carbylamine reaction in detailed form.
(i) Diazotization is the reaction in which aromatic amines that are the primary amines when treated with nitrous acid and mineral acid produce diazonium salt with the release of water as by product. The reaction in detail is as shown below:

Here, aniline is the primary amine and when this reacts with nitrous acid, it forms benzene diazonium chloride along with water as by product.
(ii) Carbylamine reaction is the one where primary amine reacts with chloroform and base to give isocyanide compounds.
The example for this reaction can be of aliphatic amines or aromatic amines as shown below:
\[\text{1) C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{N}{{\text{H}}_{2}}\text{+CHC}{{\text{l}}_{3}}\text{+3KOH}\to \text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{NC+3KCl+3}{{\text{H}}_{2}}\text{O}\]

In the above reaction 1), the aliphatic primary amine that is ethyl amine reacts with chloroform and base to give ethyl isocyanide and by product of salt and water.
Similarly, in reaction 2), primary aromatic amine that is aniline reacts with chloroform and base to give phenyl isocyanide and similar products.
Note: Note that diazotization reactions are to be carried out at lower temperatures because if we employ higher temperature then the salts form others by products and thus result in the formation of phenol by the reaction with water at higher temperature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




