
Explain the following reactions of chloroform
(i)Effect of air and sunlight (ii) Reimer-Tiemann reaction
Answer
577.8k+ views
Hint: Chloroform is a volatile, colorless, sweet-smelling liquid used as a solvent and formerly as an anesthetic. It is a man-made by-product when formed chlorine is used to disinfect the water
Complete step by step answer:
(i)Effect of air and sunlight:
Chloroform(\[CHC{{l}_{3}}\]) on the exposure of air and sunlight gives Phosgene gas and hydrochloric acid due to the oxidation of chloroform, which is used in the production of urethanes and polycarbonate plastics. In order to avoid this reaction, the chloroform is kept in dark bottles to prevent this oxidation. The reaction is given below;
\[\Rightarrow 2CHC{{l}_{3}}+{{O}_{2}}\xrightarrow[Sunlight]{Air}2COC{{l}_{2}}+HCl\]
Phosgene gas(\[COC{{l}_{2}}\]) is a colorless, poisonous gas. Formerly it used to defeat the enemies at the time of the first world war
(ii)Reimer-Tiemann reaction:
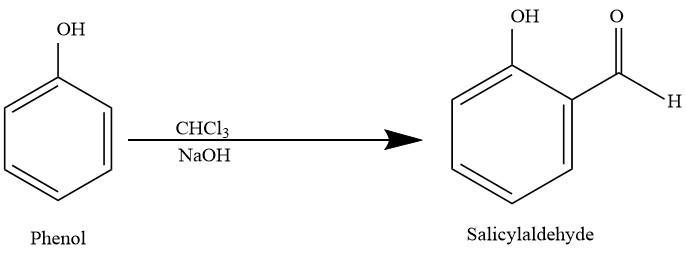
Reimer-Tiemann reaction is a type of substitution reaction. It is generally used for the ortho-formylation of phenols. The simplest example for the Reimer-Tiemann reaction is the conversion of phenol into salicylaldehyde. The reaction is discovered by Karl Reimer and Ferdinand Tiemann Reaction: When phenols is treated with chloroform in the presence of sodium hydroxide, an aldehyde group is introduced at the ortho-position of the benzene ring leading to the formation of o-hydroxybenzaldehyde(salicylaldehyde). The reaction is given below;
Note:
Hydroxide is not readily soluble in chloroform. So Reimer-tiemann's reaction is generally occurring in the bi-phasic system. Simply, an aqueous hydroxide solution phase and organic solution phase containing chloroform. These two reagents have to separate and must bring together to proceed this reaction.
Complete step by step answer:
(i)Effect of air and sunlight:
Chloroform(\[CHC{{l}_{3}}\]) on the exposure of air and sunlight gives Phosgene gas and hydrochloric acid due to the oxidation of chloroform, which is used in the production of urethanes and polycarbonate plastics. In order to avoid this reaction, the chloroform is kept in dark bottles to prevent this oxidation. The reaction is given below;
\[\Rightarrow 2CHC{{l}_{3}}+{{O}_{2}}\xrightarrow[Sunlight]{Air}2COC{{l}_{2}}+HCl\]
Phosgene gas(\[COC{{l}_{2}}\]) is a colorless, poisonous gas. Formerly it used to defeat the enemies at the time of the first world war
(ii)Reimer-Tiemann reaction:
Reimer-Tiemann reaction is a type of substitution reaction. It is generally used for the ortho-formylation of phenols. The simplest example for the Reimer-Tiemann reaction is the conversion of phenol into salicylaldehyde. The reaction is discovered by Karl Reimer and Ferdinand Tiemann Reaction: When phenols is treated with chloroform in the presence of sodium hydroxide, an aldehyde group is introduced at the ortho-position of the benzene ring leading to the formation of o-hydroxybenzaldehyde(salicylaldehyde). The reaction is given below;

Note:
Hydroxide is not readily soluble in chloroform. So Reimer-tiemann's reaction is generally occurring in the bi-phasic system. Simply, an aqueous hydroxide solution phase and organic solution phase containing chloroform. These two reagents have to separate and must bring together to proceed this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




