
Explain the formation of energy bands in solids. On the basis of energy bands distinguish between a metal, a semiconductor and an insulator.
Answer
594.6k+ views
Hint: The band theory should be understood first along with the Pauli’s exclusion principle. Then based on the difference in the energy levels of the forbidden band, the solids are distinguished in metals, semiconductors and the insulators.
Complete step by step answer:
Unlike other states of matter, the atoms in solids are very closely arranged to each other which affects the energy level of the electrons present in the outermost orbit but the neighboring atoms do not affect the electrons present in the innermost orbital. There are basically three types of bands present in a solid,
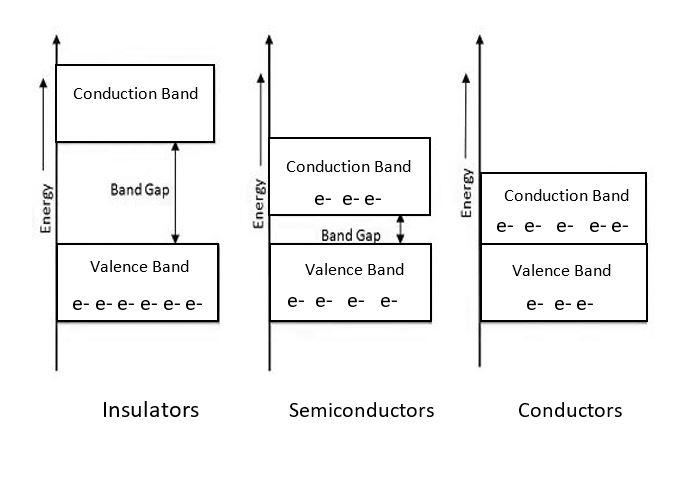
(I)Conduction band: In this, the mobile charge carriers exist having negative charge. These are simply the electrons with the energy enough to jump from the valence band to the conduction band. The electrons here move freely in the crystal lattice and are directly involved in the conductivity in metals.
(II)Forbidden band: This band is present between the valence band and the conduction band. At absolute zero, this molecular orbital is the highest occupied one.
(III)Valence band: These electrons present within the outer shell are called the valence electrons and together compose the valence band.
Now, let us understand the band theory in solids,
(I)The Pauli’s exclusion principle governs the filling of electrons in the different energy orbits
(II)A molecular orbit is formed by the combination of two atomic orbitals having distinct energy
(II)The energy band is formed by around $10^{23}$ lines stacked up in a tiny space
(IV)We can visualize the difference between the metals, insulators and the semiconductors by plotting the energies for an electron in the material.
Based on the size of the forbidden band, the solids are categorized into three categories,
(I)Metals or conductors: In these, there is no presence of forbidden energy gap and thus requires no energy for the electron to jump from the valence band to the conduction band.
(II)Semiconductors: In this, the conduction band is empty while the valence band is filled with electrons. The energy gap between the valence and conduction bands is less and electrons can make a jump in case proper energy is supplied.
(III)Insulators: In this also, the conduction band is empty while the valence band is completely filled. Because of which the energy becomes do high that the transfer of electrons from the valence band to conduction band becomes almost impossible,
Note:
The fermi energy level or the forbidden gap in case of semiconductors can be decreased when electric current is passed through it. For the transfer of electrons on semiconductors to take place, the temperature should be above 0K.
Fermi energy is used to determine the thermal and electrical properties of a solid. In nuclear physics, it is used to understand the stability of white dwarfs.
Complete step by step answer:
Unlike other states of matter, the atoms in solids are very closely arranged to each other which affects the energy level of the electrons present in the outermost orbit but the neighboring atoms do not affect the electrons present in the innermost orbital. There are basically three types of bands present in a solid,
(I)Conduction band: In this, the mobile charge carriers exist having negative charge. These are simply the electrons with the energy enough to jump from the valence band to the conduction band. The electrons here move freely in the crystal lattice and are directly involved in the conductivity in metals.
(II)Forbidden band: This band is present between the valence band and the conduction band. At absolute zero, this molecular orbital is the highest occupied one.
(III)Valence band: These electrons present within the outer shell are called the valence electrons and together compose the valence band.
Now, let us understand the band theory in solids,
(I)The Pauli’s exclusion principle governs the filling of electrons in the different energy orbits
(II)A molecular orbit is formed by the combination of two atomic orbitals having distinct energy
(II)The energy band is formed by around $10^{23}$ lines stacked up in a tiny space
(IV)We can visualize the difference between the metals, insulators and the semiconductors by plotting the energies for an electron in the material.
Based on the size of the forbidden band, the solids are categorized into three categories,
(I)Metals or conductors: In these, there is no presence of forbidden energy gap and thus requires no energy for the electron to jump from the valence band to the conduction band.
(II)Semiconductors: In this, the conduction band is empty while the valence band is filled with electrons. The energy gap between the valence and conduction bands is less and electrons can make a jump in case proper energy is supplied.
(III)Insulators: In this also, the conduction band is empty while the valence band is completely filled. Because of which the energy becomes do high that the transfer of electrons from the valence band to conduction band becomes almost impossible,

Note:
The fermi energy level or the forbidden gap in case of semiconductors can be decreased when electric current is passed through it. For the transfer of electrons on semiconductors to take place, the temperature should be above 0K.
Fermi energy is used to determine the thermal and electrical properties of a solid. In nuclear physics, it is used to understand the stability of white dwarfs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




