
Explain the hybridization involved in $PC{l_5}$ molecules.
Answer
570k+ views
Hint:As we all know that hybridization is the mixing of two atomic orbitals to give a degenerated new orbital and orbitals that are fully filled and half-filled can participate in this process. Phosphorus belong to p-block elements having electronic configuration as $n{s^2}n{p^3}$ and it forms $PC{l_5}$ with chlorine having electronic configuration $n{s^2}n{p^5}$.
Complete answer:
As we know that the concept of hybridization depends upon the mixing of two atomic orbitals having similar energies to give a degenerated new orbital or we can say that hybridization is the result of formation of a hybrid orbital formed by mixing of two atomic orbitals for redistribution of their energy and orbitals that are fully filled and half-filled can participate in this process. During mixing, the orbitals with same energy are mixed together such as the mixing of one s and one p-orbital or two s and two p-orbitals or one s or one d-orbital etc. and can be named as $sp,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2}$ etc.
Considering our molecule, using Valence Bond Theory let us first write the configurations of both elements:
In ground state, the electronic configuration of phosphorus is, P= $3{s^2}3{p^3}$ and Cl=$3{s^2}3{p^5}$
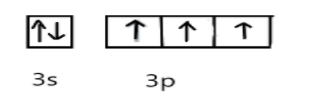
In excited state, under the conditions of bond formation electron in s-orbitals get unpaired and one electron will be promoted to vacant d-orbital as shown:
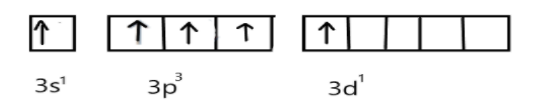
Now, these five singly occupied orbitals will overlap with the 3pz-orbitals of five chlorine atoms and form five σ-bonds between P-C:
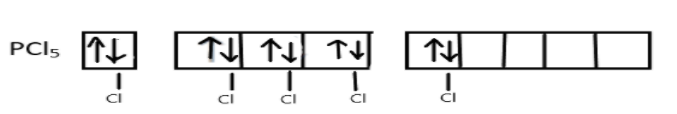
Hence, the final result will be a $s{p^3}d$ hybridized molecule $PC{l_5}$ involving one s, three p and one d-orbital. The geometry of this molecule is trigonal bipyramidal, three of the hybrid orbitals lie in a horizontal plane at an angle of ${120^ \circ }$ to one another and other two orbitals will lie in a vertical plane at right angle to the horizontal orbitals.
Note:
The hybrid state of some atoms like $I{F_5},S{F_4},ClO_3^ - $ can be easily found using the formula: $X = SA + \dfrac{1}{2}(G - V) $ where SA is the number of atoms surrounding the central atom, G is valence electrons of central atom and V is valency of central atom.
Complete answer:
As we know that the concept of hybridization depends upon the mixing of two atomic orbitals having similar energies to give a degenerated new orbital or we can say that hybridization is the result of formation of a hybrid orbital formed by mixing of two atomic orbitals for redistribution of their energy and orbitals that are fully filled and half-filled can participate in this process. During mixing, the orbitals with same energy are mixed together such as the mixing of one s and one p-orbital or two s and two p-orbitals or one s or one d-orbital etc. and can be named as $sp,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2}$ etc.
Considering our molecule, using Valence Bond Theory let us first write the configurations of both elements:
In ground state, the electronic configuration of phosphorus is, P= $3{s^2}3{p^3}$ and Cl=$3{s^2}3{p^5}$

In excited state, under the conditions of bond formation electron in s-orbitals get unpaired and one electron will be promoted to vacant d-orbital as shown:

Now, these five singly occupied orbitals will overlap with the 3pz-orbitals of five chlorine atoms and form five σ-bonds between P-C:

Hence, the final result will be a $s{p^3}d$ hybridized molecule $PC{l_5}$ involving one s, three p and one d-orbital. The geometry of this molecule is trigonal bipyramidal, three of the hybrid orbitals lie in a horizontal plane at an angle of ${120^ \circ }$ to one another and other two orbitals will lie in a vertical plane at right angle to the horizontal orbitals.
Note:
The hybrid state of some atoms like $I{F_5},S{F_4},ClO_3^ - $ can be easily found using the formula: $X = SA + \dfrac{1}{2}(G - V) $ where SA is the number of atoms surrounding the central atom, G is valence electrons of central atom and V is valency of central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




