
Explain the preparation of acetic acid by a quick vinegar process with diagram.
Answer
585k+ views
Hint: Vinegar is a liquid consisting of about $5 - 20\% $ acetic acid ( $C{H_3}COOH$ ), water and other chemicals. The acetic acid is produced by the fermentation of ethanol by the acetic acid enzyme.
Complete step by step solution:
There are three methods used for the production of vinegar: Orleans Method (also known as the slow method), Trickling/German (or quick) Method and Submerged Fermentation.
We are going to learn about the Quick Vinegar Method in detail,
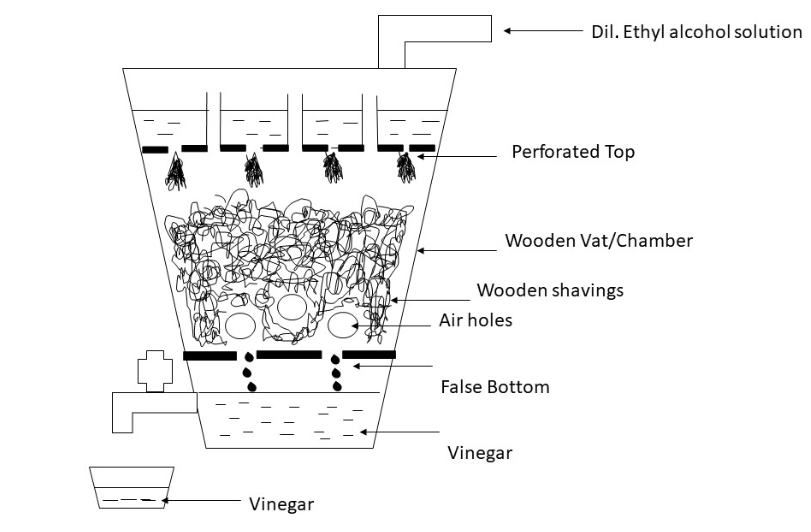
In German/Trickle method also known as quick method a generator is used, which is a tank filled with beech wood shavings and devices are assembled which allows the dilute aqueous solution of ethyl alcohol to trickle down through the wood shavings in which the acetic acid enzymes are living, these enzymes are Mycoderma aceti, , and the tank or wooden vat/chamber is not filled totally as that would leave no space for oxygen which is necessary for the fermentation. Near the bottom of the generator there are holes known as the air holes as they allow air to penetrate in. The air rises through the generator and is used by the acetic acid enzyme to oxidise the alcohol. This oxidation also releases considerable amounts of heat which must be controlled so that it does not cause any damage to the enzymes present.
The reaction involves oxidation of ethyl alcohol under enzyme to form acetic acid,
$C{H_3}C{H_2}OH\xrightarrow[{}]{{{O_2}}}C{H_3}COOH + {H_2}O$
The labelled diagram is as follows,
Additional Information: Vinegar is mainly used as a cooking ingredient, or in pickling. As it is one of the most easily manufactured acids, it has a great value when it comes to industrial, medical, and domestic uses, as it is used as a household cleaner till date.
Note: The aqueous solution of ethyl alcohol added in the process ranges from $8 - 12\% $ , and the student can confuse about the fact that how is oxygen necessary for fermentation as the fermentation process is anaerobic this is because in the manufacturing of vinegar there are two stages of fermentation one is anaerobic and the second stage is aerobic which involves the oxidation of ethanol to ethanoic acid.
Complete step by step solution:
There are three methods used for the production of vinegar: Orleans Method (also known as the slow method), Trickling/German (or quick) Method and Submerged Fermentation.
We are going to learn about the Quick Vinegar Method in detail,
In German/Trickle method also known as quick method a generator is used, which is a tank filled with beech wood shavings and devices are assembled which allows the dilute aqueous solution of ethyl alcohol to trickle down through the wood shavings in which the acetic acid enzymes are living, these enzymes are Mycoderma aceti, , and the tank or wooden vat/chamber is not filled totally as that would leave no space for oxygen which is necessary for the fermentation. Near the bottom of the generator there are holes known as the air holes as they allow air to penetrate in. The air rises through the generator and is used by the acetic acid enzyme to oxidise the alcohol. This oxidation also releases considerable amounts of heat which must be controlled so that it does not cause any damage to the enzymes present.
The reaction involves oxidation of ethyl alcohol under enzyme to form acetic acid,
$C{H_3}C{H_2}OH\xrightarrow[{}]{{{O_2}}}C{H_3}COOH + {H_2}O$
The labelled diagram is as follows,

Additional Information: Vinegar is mainly used as a cooking ingredient, or in pickling. As it is one of the most easily manufactured acids, it has a great value when it comes to industrial, medical, and domestic uses, as it is used as a household cleaner till date.
Note: The aqueous solution of ethyl alcohol added in the process ranges from $8 - 12\% $ , and the student can confuse about the fact that how is oxygen necessary for fermentation as the fermentation process is anaerobic this is because in the manufacturing of vinegar there are two stages of fermentation one is anaerobic and the second stage is aerobic which involves the oxidation of ethanol to ethanoic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




