
Explain the structure of carbon dioxide?
Answer
509.4k+ views
Hint :The chemical formula of carbon dioxide is $ C{O_2} $ .It is a colourless and odourless gas in nature. It consists of carbon atoms covalently bonded to oxygen atoms.
Complete Step By Step Answer:
Carbon dioxide molecules consist of one carbon atom and two oxygen atoms. It has two double bonds between the carbon and oxygen atom. Each double bond is made of one sigma and one pi bond. In all the carbon dioxide molecule contains two sigma and two pi bonds.
The structure is as follows:
$ O = C = O $
The bond angle is $ {180^0} $ between both the carbon and the two oxygen atoms. Hence, Carbon dioxide has a linear structure.
The carbon atom undergoes $ sp $ hybridization and both oxygen atoms are $ s{p^2} $ hybridized. Carbon dioxide is a covalent compound
The two bonds formed are polar as oxygen is more electronegative than carbon. But due to cancellation of the dipole moment, the molecule as a whole is not polar.
The Lewis structure of carbon dioxide is as follows:
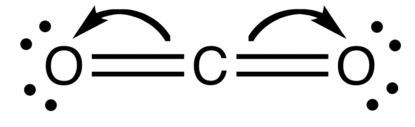
The bonds appear as parallel lines between carbon and oxygen atoms.
Properties of Carbon dioxide:
-Carbon dioxide is a colourless and odourless gas in nature.
-The density of carbon dioxide is $ 53\% $ higher than that of air.
Note :
Carbon dioxide makes up less than $ 1\% $ in the atmosphere, nevertheless it is an important greenhouse gas. Combustion of petrol and diesel in cars, respiration of living things increases the amount of carbon dioxide in air. Carbon dioxide finds its applications as a refrigerant, fire extinguishers, foaming rubbers.
Complete Step By Step Answer:
Carbon dioxide molecules consist of one carbon atom and two oxygen atoms. It has two double bonds between the carbon and oxygen atom. Each double bond is made of one sigma and one pi bond. In all the carbon dioxide molecule contains two sigma and two pi bonds.
The structure is as follows:
$ O = C = O $
The bond angle is $ {180^0} $ between both the carbon and the two oxygen atoms. Hence, Carbon dioxide has a linear structure.
The carbon atom undergoes $ sp $ hybridization and both oxygen atoms are $ s{p^2} $ hybridized. Carbon dioxide is a covalent compound
The two bonds formed are polar as oxygen is more electronegative than carbon. But due to cancellation of the dipole moment, the molecule as a whole is not polar.
The Lewis structure of carbon dioxide is as follows:
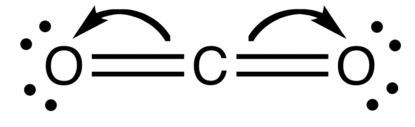
The bonds appear as parallel lines between carbon and oxygen atoms.
Properties of Carbon dioxide:
-Carbon dioxide is a colourless and odourless gas in nature.
-The density of carbon dioxide is $ 53\% $ higher than that of air.
Note :
Carbon dioxide makes up less than $ 1\% $ in the atmosphere, nevertheless it is an important greenhouse gas. Combustion of petrol and diesel in cars, respiration of living things increases the amount of carbon dioxide in air. Carbon dioxide finds its applications as a refrigerant, fire extinguishers, foaming rubbers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




