
Explain the structure of Graphite.
Answer
501k+ views
Hint: Graphite, sometimes known as plumbago, is a crystalline form of the element carbon with atoms organised in a hexagonal pattern. It is the most stable form of carbon under normal circumstances and occurs naturally in this form. It turns to diamond under high pressures and temperatures. Graphite is a mineral that is found in pencils and lubricants. It is a good heat and electrical conductor. Its strong conductivity makes it ideal for electrodes, batteries, and solar panels.
Complete answer:
The structure of this crystal carbon is flat and layered. Each layer of graphene is referred to as a graphene layer.
Every layer has carbon atoms organised in a honeycomb-like network with a division of 0.142 nm and a spacing between planes of 0.335 nm. The structure of graphite is shown below.
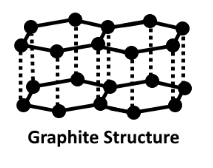
For atoms in the plane, there is covalent bonding, but only three out of four possible bonding sites meet the requirements.
Because the fourth electron has a chance to move into the plane, the graphite would be electrically favourable.
The layers of the carbon crystal may travel past each other quickly because the layers could be readily separated by weak van der Waals connections.
Each carbon atom in graphite develops a sheet-like structure by forming a covalent connection with three neighbouring carbon atoms. To create a three-dimensional structure, these sheets or layers are layered one on top of the other. There is no covalent connection between the layers since they are built up of hexagons. The physical forces of Vander Wal hold these layers together. As a result, these layers can be stacked on top of one another.
Note:
Graphite is used in a variety of applications today, including steelmaking, brake linings, lubricants, foundry facings, and batteries, to mention a few. Graphene, one of the most significant components of graphite, has unique properties and is one of the most well-known strong materials. Separating the component from the carbon crystal would necessitate more technological advancements. Electrodes and refractories used in high-temperature processing applications are among the crystal's applications.
Complete answer:
The structure of this crystal carbon is flat and layered. Each layer of graphene is referred to as a graphene layer.
Every layer has carbon atoms organised in a honeycomb-like network with a division of 0.142 nm and a spacing between planes of 0.335 nm. The structure of graphite is shown below.

For atoms in the plane, there is covalent bonding, but only three out of four possible bonding sites meet the requirements.
Because the fourth electron has a chance to move into the plane, the graphite would be electrically favourable.
The layers of the carbon crystal may travel past each other quickly because the layers could be readily separated by weak van der Waals connections.
Each carbon atom in graphite develops a sheet-like structure by forming a covalent connection with three neighbouring carbon atoms. To create a three-dimensional structure, these sheets or layers are layered one on top of the other. There is no covalent connection between the layers since they are built up of hexagons. The physical forces of Vander Wal hold these layers together. As a result, these layers can be stacked on top of one another.
Note:
Graphite is used in a variety of applications today, including steelmaking, brake linings, lubricants, foundry facings, and batteries, to mention a few. Graphene, one of the most significant components of graphite, has unique properties and is one of the most well-known strong materials. Separating the component from the carbon crystal would necessitate more technological advancements. Electrodes and refractories used in high-temperature processing applications are among the crystal's applications.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




