
Explain the working of Daniel cell with a neat diagram.
Answer
568.8k+ views
Hint: We know that there are several types of cells used in different experiments with different purposes. We can understand the mechanism the Daniel cell uses in order to produce an electromotive force that can trigger the flow of charges.
Complete answer:
Daniel cell is a primary cell which produces an electromotive force between its two terminals by a series of chemical reactions. It is essentially made up of copper and zinc with the cuprite sulphate as an electrolyte. We can understand from these that the Daniel cell is a galvanic cell that converts the chemical energy into the chemical energy.
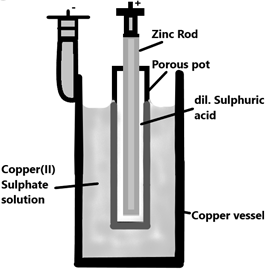
The Daniel comprises a copper vessel with copper (II) sulphate solution which acts as the anode and a zinc rod which is dipped in dilute sulphuric acid which is kept in a porous pot which acts as the cathode. This arrangement of porous pot is kept within the copper vessel thus forming a cylindrical structure. The Zinc atoms on the rod are oxidised and the copper ions in the copper sulphate solution reduces and gets deposited on the outer copper vessel.
The cell reactions are –
At cathode: \[Z{{n}_{(s)}}\to Z{{n}^{2+}}_{(aq)}+2{{e}^{-}}\]
At anode: \[C{{u}^{2+}}_{(aq)}+2{{e}^{-}}\to C{{u}_{(s)}}\]
Total cell reaction becomes: \[Z{{n}_{(s)}}+C{{u}^{2+}}_{(aq)}\to Z{{n}^{2+}}_{(aq)}+C{{u}_{(s)}}\]
The cell emf produced by a Daniel cell is 1.1018V. This Daniel cell exhausts when all the zinc in the cathode gets ionised. As a result, the battery becomes dead after some time.
This is the required solution.
Note:
The Daniel cells are the common cells that we use for the domestic and household purposes. We have the AA and AAA batteries which are used in the toys, clocks and the remotes which are the basic primary voltaic cells used in our households.
Complete answer:
Daniel cell is a primary cell which produces an electromotive force between its two terminals by a series of chemical reactions. It is essentially made up of copper and zinc with the cuprite sulphate as an electrolyte. We can understand from these that the Daniel cell is a galvanic cell that converts the chemical energy into the chemical energy.

The Daniel comprises a copper vessel with copper (II) sulphate solution which acts as the anode and a zinc rod which is dipped in dilute sulphuric acid which is kept in a porous pot which acts as the cathode. This arrangement of porous pot is kept within the copper vessel thus forming a cylindrical structure. The Zinc atoms on the rod are oxidised and the copper ions in the copper sulphate solution reduces and gets deposited on the outer copper vessel.
The cell reactions are –
At cathode: \[Z{{n}_{(s)}}\to Z{{n}^{2+}}_{(aq)}+2{{e}^{-}}\]
At anode: \[C{{u}^{2+}}_{(aq)}+2{{e}^{-}}\to C{{u}_{(s)}}\]
Total cell reaction becomes: \[Z{{n}_{(s)}}+C{{u}^{2+}}_{(aq)}\to Z{{n}^{2+}}_{(aq)}+C{{u}_{(s)}}\]
The cell emf produced by a Daniel cell is 1.1018V. This Daniel cell exhausts when all the zinc in the cathode gets ionised. As a result, the battery becomes dead after some time.
This is the required solution.
Note:
The Daniel cells are the common cells that we use for the domestic and household purposes. We have the AA and AAA batteries which are used in the toys, clocks and the remotes which are the basic primary voltaic cells used in our households.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




