
Explain the Wurtz-Fittig reaction.
Answer
596.1k+ views
Hint: This reaction is very similar to Wurtz reaction and Fittig reaction. A metal is used as a reagent and dry ether is used as a solvent in this reaction. The product from this reaction is aromatic in nature and has alkyl side chains.
Complete Step-by-Step Solution:
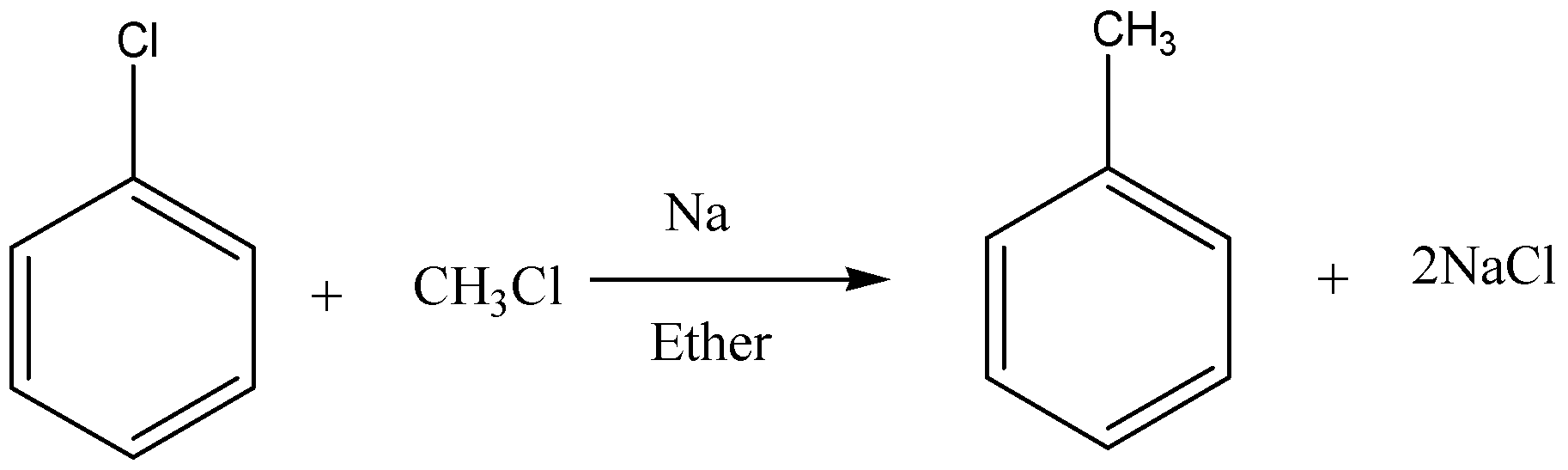
Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Reaction can be written as under.
\[Ar - X + R - X\xrightarrow[{Ether}]{{Na}}Ar - R + 2NaX\]
So, as shown here an aromatic alkane is produced with this reaction. Sodium salt is produced as a byproduct.
- We can use any aromatic and any alkyl group here in this reaction. We generally use chlorine and bromine as halides as they are easily available however, other halogens also give this reaction.
- Ether is used as a solvent in this reaction. The ether we use here is required to be dry as we are using metallic sodium here.
Below is an example of Wurtz-Fittig reaction.
Additional Information:
We also have two other similar reactions like Wurtz-Fittig reaction that differ in just starting materials.
Wurtz reaction :
It has the same reagents like Wurtz-Fittig reaction but it involves two alkyl halides reacting in this reaction to give an alkane. The reaction is shown below.
\[C{H_3}C{H_2} - Cl + C{H_3} - Cl\xrightarrow[{Ether}]{{Na}}C{H_3}C{H_2}C{H_3} + 2NaCl\]
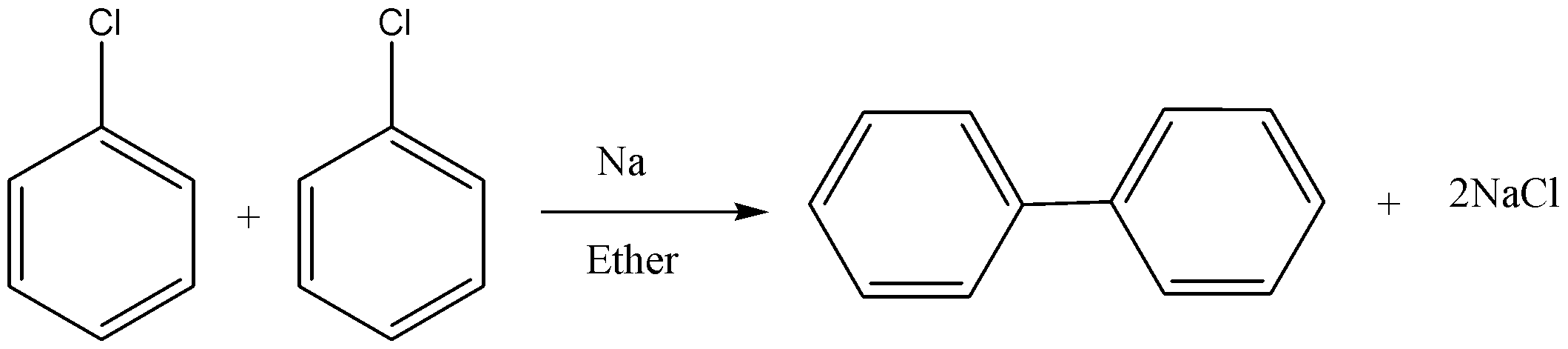
Fittig reaction :
In this reaction, two aryl halides react to give biaryl compounds in presence of sodium and dry ether. The reaction is shown below.
Note:
Remember that there are very narrow differences between Wurtz, Wurtz-Fittig and Fittig reactions, so do not get confused with that. Remember that halogen present in halide compounds here is not removed as halide gas but it is removed in the form of sodium salt.
Complete Step-by-Step Solution:
Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Reaction can be written as under.
\[Ar - X + R - X\xrightarrow[{Ether}]{{Na}}Ar - R + 2NaX\]
So, as shown here an aromatic alkane is produced with this reaction. Sodium salt is produced as a byproduct.
- We can use any aromatic and any alkyl group here in this reaction. We generally use chlorine and bromine as halides as they are easily available however, other halogens also give this reaction.
- Ether is used as a solvent in this reaction. The ether we use here is required to be dry as we are using metallic sodium here.
Below is an example of Wurtz-Fittig reaction.

Additional Information:
We also have two other similar reactions like Wurtz-Fittig reaction that differ in just starting materials.
Wurtz reaction :
It has the same reagents like Wurtz-Fittig reaction but it involves two alkyl halides reacting in this reaction to give an alkane. The reaction is shown below.
\[C{H_3}C{H_2} - Cl + C{H_3} - Cl\xrightarrow[{Ether}]{{Na}}C{H_3}C{H_2}C{H_3} + 2NaCl\]
Fittig reaction :
In this reaction, two aryl halides react to give biaryl compounds in presence of sodium and dry ether. The reaction is shown below.

Note:
Remember that there are very narrow differences between Wurtz, Wurtz-Fittig and Fittig reactions, so do not get confused with that. Remember that halogen present in halide compounds here is not removed as halide gas but it is removed in the form of sodium salt.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




