
Explain three examples of chiral and Achiral compounds?
Answer
503.4k+ views
Hint: Compounds which have capability to rotate plane polarized light either in right direction or left direction are known as optical isomers. A compound to be optically active must possess one or more chiral centres.
Complete answer:
Centre of a compound which is attached to different types of substituent is known as chiral Centre and compounds which possess one or more such chiral centres are known as chiral compounds.
When a carbon atom in a molecule shares its all the four bonds with different types of substituents are known as chiral carbon.
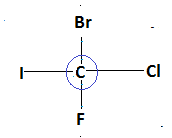
In the given compound as we see that carbon shares its valency with four different types of atom and hence form a chiral compound.
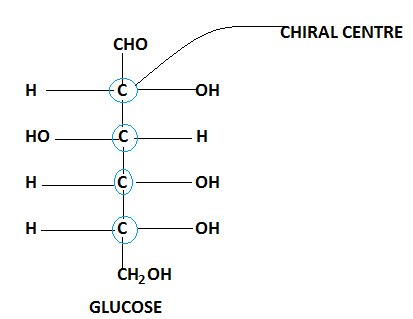
Glucose is composed of six carbon atoms out of which four atoms are chiral as represented as blue circles in the given diagram.
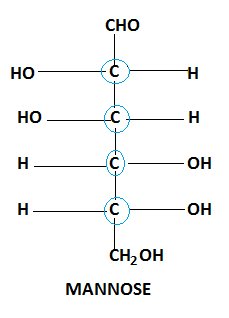
Similarly, molecules of Mannose form chiral compounds with a total four chiral centres.
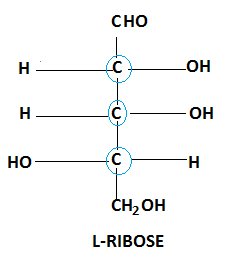
Molecule of Ribose also shows chirality as it has a total number of three chiral centres.
Centre of a compound which is attached to similar types of substituent is known as achiral Centre and compounds which lack any chiral Centre are known as achiral compounds.
When a carbon atom in a molecule shares its bond with similar types of substituents are known as achiral carbon.
Presence of minimum two similar substituent to the carbon atom can make it achiral Centre.
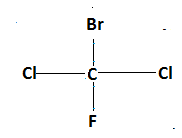
As we see in the above molecule, a carbon atom is attached to two chlorine atoms hence, it is achiral compound.
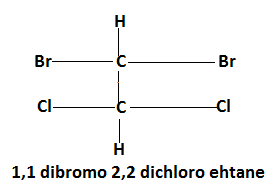
Molecule of $1,1$ dibromo $2,2$ dichloromethane shows Achirality because two similar groups are added to a single carbon atom.
Achirality in molecules also appears when a compound is divided into identical halves. For example, $1,2$dibromo cinnamic acid.
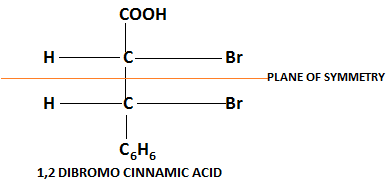
In the above molecule carbon is attached to different atoms but the entire compound is divided into two equal halves therefore it is achiral compound.
The line which divides the compound into two identical halves is known as the plane of symmetry.
Note:
Optically active compounds which are mirror images to each other are defined as Enantiomers. Compounds must be chiral to show the property of enantiomers.
These enantiomers have different biological properties hence only enzymes are capable of separating one mirror image from another.
Complete answer:
Centre of a compound which is attached to different types of substituent is known as chiral Centre and compounds which possess one or more such chiral centres are known as chiral compounds.
When a carbon atom in a molecule shares its all the four bonds with different types of substituents are known as chiral carbon.

In the given compound as we see that carbon shares its valency with four different types of atom and hence form a chiral compound.
Glucose is composed of six carbon atoms out of which four atoms are chiral as represented as blue circles in the given diagram.

Similarly, molecules of Mannose form chiral compounds with a total four chiral centres.

Molecule of Ribose also shows chirality as it has a total number of three chiral centres.

Centre of a compound which is attached to similar types of substituent is known as achiral Centre and compounds which lack any chiral Centre are known as achiral compounds.
When a carbon atom in a molecule shares its bond with similar types of substituents are known as achiral carbon.
Presence of minimum two similar substituent to the carbon atom can make it achiral Centre.

As we see in the above molecule, a carbon atom is attached to two chlorine atoms hence, it is achiral compound.
Molecule of $1,1$ dibromo $2,2$ dichloromethane shows Achirality because two similar groups are added to a single carbon atom.

Achirality in molecules also appears when a compound is divided into identical halves. For example, $1,2$dibromo cinnamic acid.

In the above molecule carbon is attached to different atoms but the entire compound is divided into two equal halves therefore it is achiral compound.
The line which divides the compound into two identical halves is known as the plane of symmetry.
Note:
Optically active compounds which are mirror images to each other are defined as Enantiomers. Compounds must be chiral to show the property of enantiomers.
These enantiomers have different biological properties hence only enzymes are capable of separating one mirror image from another.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




