
Find out the bond order in ${{N}_{2}}$ molecule and also draw the energy level diagram.
Answer
573.9k+ views
Hint: Bond order is introduced by Linus Pauling, . The bond number itself is that the number of electron pairs (bonds) between a pair of atoms. Bond number gives a sign of the steadiness of a bond. Isoelectronic species have the same bond number.
Complete step by step Solution:
If there are greater than two atoms within the molecule, follow these steps to see the bond order: Draw the Lewis structure. Count the entire number of bonds. Count the amount of bond groups between individual atoms. Divide the amount of bonds between atoms by the overall number of bond groups within the molecule. Bond order is defined as half the difference between the amount of electrons in bonding molecular orbital (${{N}_{b}}$) and therefore the number of electrons within the antibonding molecular orbitals (${{N}_{a}}$). The bond order describes the steadiness of the bond. The molecular orbital provides a simple understanding of the concept of the bond order of an attraction. It gives us an estimated quantity of the degree of covalent bonds between the atoms. i.e., Bond order =$\dfrac{1}{2}({{N}_{b}}-{{N}_{a}})$
Here ${{N}_{b}}$ is the no of bonding electrons and ${{N}_{a}}$ is the no of antibonding electrons. Number of bonds during a molecule is additionally referred to as bond order.
Energy diagram of ${{N}_{2}}$ is:
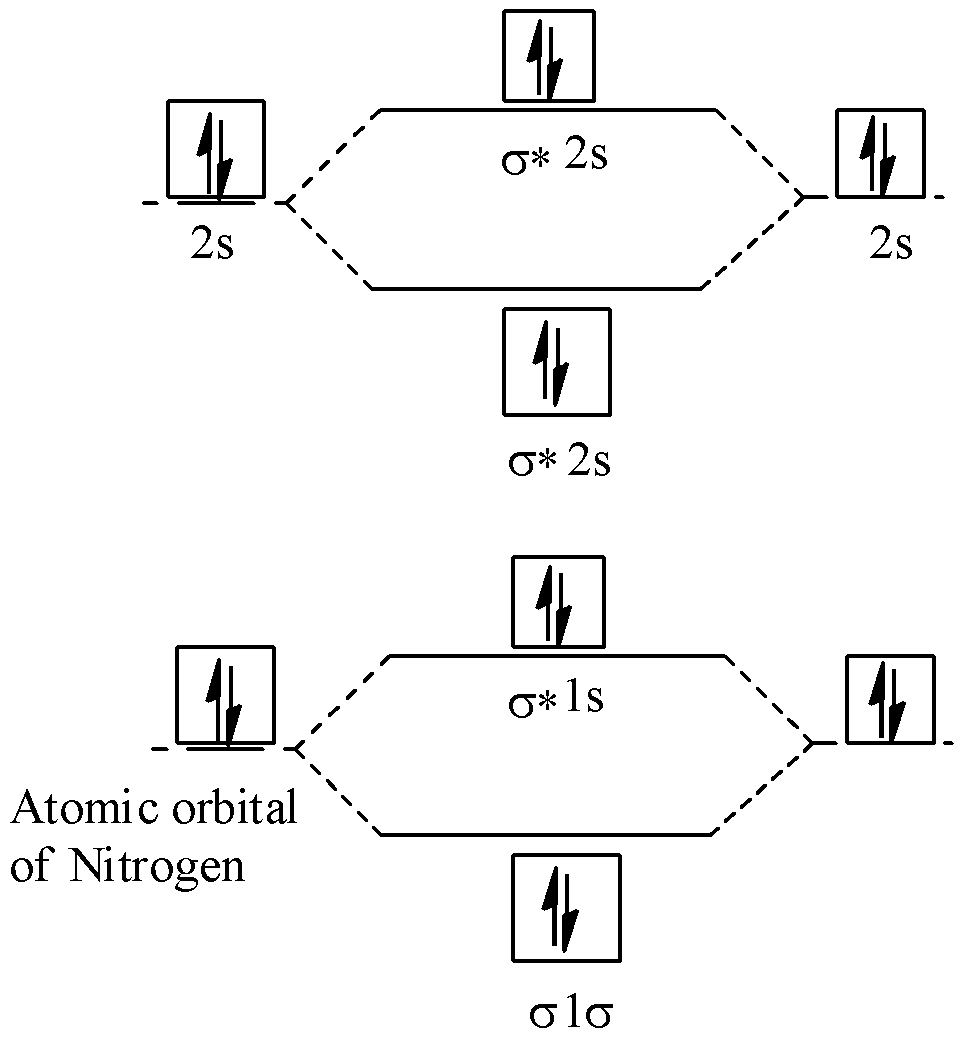
Bond order of ${{N}_{2}}=\dfrac{1}{2}(10-4)=\dfrac{1}{2}\times 6=3$, so it has 3 bonds.
Note: If there are unpaired electrons present in the orbitals then it is paramagnetic otherwise diamagnetic. The length of the bond is decided by the quantity of bonded electrons (the bond order). the upper the bond order, the stronger the pull between the 2 atoms and also the shorter the bond length. Generally, the covalent radii of the two atoms gives us the approximate length between the bonds.
Complete step by step Solution:
If there are greater than two atoms within the molecule, follow these steps to see the bond order: Draw the Lewis structure. Count the entire number of bonds. Count the amount of bond groups between individual atoms. Divide the amount of bonds between atoms by the overall number of bond groups within the molecule. Bond order is defined as half the difference between the amount of electrons in bonding molecular orbital (${{N}_{b}}$) and therefore the number of electrons within the antibonding molecular orbitals (${{N}_{a}}$). The bond order describes the steadiness of the bond. The molecular orbital provides a simple understanding of the concept of the bond order of an attraction. It gives us an estimated quantity of the degree of covalent bonds between the atoms. i.e., Bond order =$\dfrac{1}{2}({{N}_{b}}-{{N}_{a}})$
Here ${{N}_{b}}$ is the no of bonding electrons and ${{N}_{a}}$ is the no of antibonding electrons. Number of bonds during a molecule is additionally referred to as bond order.
Energy diagram of ${{N}_{2}}$ is:

Bond order of ${{N}_{2}}=\dfrac{1}{2}(10-4)=\dfrac{1}{2}\times 6=3$, so it has 3 bonds.
Note: If there are unpaired electrons present in the orbitals then it is paramagnetic otherwise diamagnetic. The length of the bond is decided by the quantity of bonded electrons (the bond order). the upper the bond order, the stronger the pull between the 2 atoms and also the shorter the bond length. Generally, the covalent radii of the two atoms gives us the approximate length between the bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




