
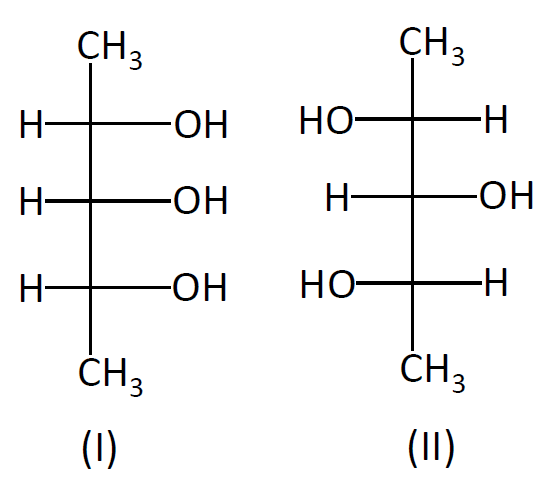
Find out the relation between I and II
A. Enantiomers
B. Diastereomers
C. Homomers
D. Structural isomers

Answer
595.2k+ views
Hint: Stereoisomers are compounds of the same molecular formula but the arrangement of the groups to carbon atoms relative to each other are different. If we place a mirror in between above compounds we see that they are not mirror images and thus they are diastereomers.
Complete step by step solution:
We have been given two organic compounds with the same molecular groups but different arrangements with respect to each other. Such compounds are known as stereoisomers. In stereoisomers, we have two forms: enantiomers and diastereomers. Enantiomers are optical isomers and they are related to each other when they are mirror images of each other. Please note that representation of compounds is shown in two dimensions but carbon atoms form covalent bonds in tetrahedral. So spatial arrangements of the atom do matter when reactivity is considered and thus above mentioned classification came under study. When the above mentioned reflection criteria does not relate the compounds then they are called as diastereomers. So above given compounds are diastereomers. We can say that option B is correct. In structural isomerism, the groups are bonded to different carbon atoms in order. This is not true for the above given compounds. Homomer is not related to the polymers but different representations and above examples are polymers with hydroxyl groups in different planes.
Note: It is better in such a problem to be thorough with the different definitions. Test the given compounds with each definition and then reduce to the correct option. Many times two options, like here A and B are two types of stereoisomers. If one is ticked, the other is straightway crossed out. Therefore, thorough definition will help to solve the problem.
Complete step by step solution:
We have been given two organic compounds with the same molecular groups but different arrangements with respect to each other. Such compounds are known as stereoisomers. In stereoisomers, we have two forms: enantiomers and diastereomers. Enantiomers are optical isomers and they are related to each other when they are mirror images of each other. Please note that representation of compounds is shown in two dimensions but carbon atoms form covalent bonds in tetrahedral. So spatial arrangements of the atom do matter when reactivity is considered and thus above mentioned classification came under study. When the above mentioned reflection criteria does not relate the compounds then they are called as diastereomers. So above given compounds are diastereomers. We can say that option B is correct. In structural isomerism, the groups are bonded to different carbon atoms in order. This is not true for the above given compounds. Homomer is not related to the polymers but different representations and above examples are polymers with hydroxyl groups in different planes.
Note: It is better in such a problem to be thorough with the different definitions. Test the given compounds with each definition and then reduce to the correct option. Many times two options, like here A and B are two types of stereoisomers. If one is ticked, the other is straightway crossed out. Therefore, thorough definition will help to solve the problem.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




