
Find the number of acids which are having peroxy linkage from the following:
${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}$,${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$,${\text{HCl}}{{\text{O}}_{\text{4}}}$
Answer
576.9k+ views
Hint: The $\left( { - {\text{O}} - {\text{O}} - } \right)$ is known as peroxy linkage. If direct attachment of oxygen with the central atom, causes the oxidation state of the central atom more than its maximum oxidation state then peroxy linkage forms.
Complete step by step answer:
${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$ is known as peroxy phosphoric acid. The oxidation state of the phosphorus in peroxy phosphoric acid is $ + 5$. Phosphorus has one double-bonded oxygen atom, two hydroxyl groups and one peroxy linkage.
${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ is known as peroxomonosulphuric acid. It is also known as Caro’s acid. The oxidation state of the sulphur in peroxomonosulphuric acid is $ + 6$. Sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}$ is known as pyrosulphuric acid. It is also known as oleum acid. The oxidation state of both of the sulphur in pyrosulphuric acid is $ + 6$. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one sulphur-oxygen-sulphur linkage.
${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$ is known as peroxodisulphuric acid. It is also known as Marshall’s acid. The oxidation state of both of the sulphur in peroxodisulphuric acid is $ + 6$. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
${\text{HCl}}{{\text{O}}_{\text{4}}}$ is known as perchloric acid. The oxidation state of chlorine in perchloric acid is $ + 7$. Chlorine has three double-bonded oxygen atoms and one hydroxyl group.
The structures of all acids are as follows:
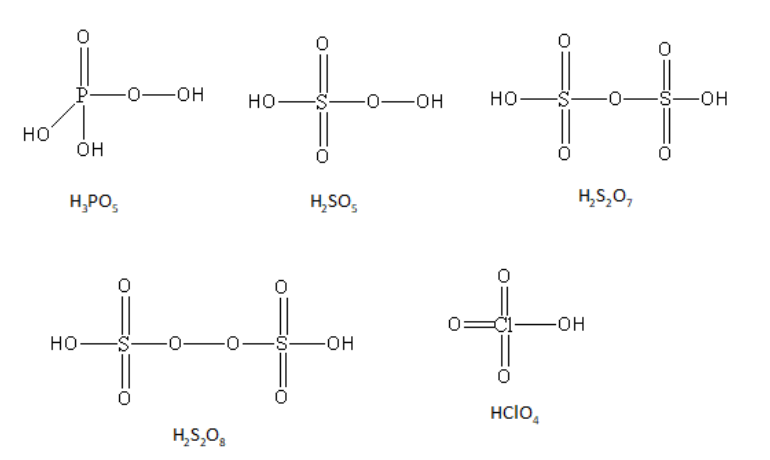
So, the peroxy linkage is present in ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ and ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$.
Therefore, the acids ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ and ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$ all three have a peroxy linkage.
Note:
The acids that have peroxy linkage can be identified by the names. The name of acid will start from ‘peroxy’ if the acid has peroxy linkage. ${\text{O}}_2^{2 - }$ is known as peroxide, so this linkage is known as peroxy. The acid which forms by heating of the acid that has a central atom in its maximum oxidation state is known as pyro acid. The pyrosulphuric acid ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}$is forms by heating two molecules of sulphuric acid.
Complete step by step answer:
${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$ is known as peroxy phosphoric acid. The oxidation state of the phosphorus in peroxy phosphoric acid is $ + 5$. Phosphorus has one double-bonded oxygen atom, two hydroxyl groups and one peroxy linkage.
${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ is known as peroxomonosulphuric acid. It is also known as Caro’s acid. The oxidation state of the sulphur in peroxomonosulphuric acid is $ + 6$. Sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}$ is known as pyrosulphuric acid. It is also known as oleum acid. The oxidation state of both of the sulphur in pyrosulphuric acid is $ + 6$. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one sulphur-oxygen-sulphur linkage.
${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$ is known as peroxodisulphuric acid. It is also known as Marshall’s acid. The oxidation state of both of the sulphur in peroxodisulphuric acid is $ + 6$. Each sulphur has two double-bonded oxygen atoms, one hydroxyl group and one peroxy linkage.
${\text{HCl}}{{\text{O}}_{\text{4}}}$ is known as perchloric acid. The oxidation state of chlorine in perchloric acid is $ + 7$. Chlorine has three double-bonded oxygen atoms and one hydroxyl group.
The structures of all acids are as follows:

So, the peroxy linkage is present in ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ and ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$.
Therefore, the acids ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{5}}}$,${{\text{H}}_2}{\text{S}}{{\text{O}}_{\text{5}}}$ and ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_8}$ all three have a peroxy linkage.
Note:
The acids that have peroxy linkage can be identified by the names. The name of acid will start from ‘peroxy’ if the acid has peroxy linkage. ${\text{O}}_2^{2 - }$ is known as peroxide, so this linkage is known as peroxy. The acid which forms by heating of the acid that has a central atom in its maximum oxidation state is known as pyro acid. The pyrosulphuric acid ${{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}$is forms by heating two molecules of sulphuric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




